3–2C What is the difference between saturated liquid and compressed liquid? Get 3.2 exercise solution
3–3C What is the difference between saturated vapor and superheated vapor? Get 3.3 exercise solution
3–4C Is there any difference between the intensive properties of saturated vapor at a given temperature and the vapor of a saturated mixture at the same temperature? Get 3.4 exercise solution
3–5C Is there any difference between the intensive properties of saturated liquid at a given temperature and the liquid of a saturated mixture at the same temperature? Get 3.5 exercise solution
3–6C Is it true that water boils at higher temperatures at higher pressures? Explain. Get 3.6 exercise solution
3–7C If the pressure of a substance is increased during a boiling process, will the temperature also increase or will it remain constant? Why? Get 3.7 exercise solution
3–8C Why are the temperature and pressure dependent properties in the saturated mixture region? Get 3.8 exercise solution
3–9C What is the difference between the critical point and the triple point? Get 3.9 exercise solution
3–10C Is it possible to have water vapor at -10°C? Get 3.10 exercise solution
3–11C A househusband is cooking beef stew for his family in a pan that is (a) uncovered, (b) covered with a light lid, and (c) covered with a heavy lid. For which case will the cooking time be the shortest? Why? Get 3.11 exercise solution
3–12C How does the boiling process at supercritical pressures differ from the boiling process at subcritical pressures? Property Tables Get 3.12 exercise solution
3–13C In what kind of pot will a given volume of water boil at a higher temperature: a tall and narrow one or a short and wide one? Explain. Get 3.13 exercise solution
3–14C A perfectly fitting pot and its lid often stick after cooking, and it becomes very difficult to open the lid when the pot cools down. Explain why this happens and what you would do to open the lid. Get 3.14 exercise solution
3–15C It is well known that warm air in a cooler environment rises. Now consider a warm mixture of air and gasoline on top of an open gasoline can. Do you think this gas mixture will rise in a cooler environment? Get 3.15 exercise solution
3–16C In 1775, Dr. William Cullen made ice in Scotland by evacuating the air in a water tank. Explain how that device works, and discuss how the process can be made more efficient. Get 3.16 exercise solution
3–17C Does the amount of heat absorbed as 1 kg of saturated liquid water boils at 100°C have to be equal to the amount of heat released as 1 kg of saturated water vapor condenses at 100°C? Get 3.17 exercise solution
3–18C Does the reference point selected for the properties of a substance have any effect on thermodynamic analysis? Why? Get 3.18 exercise solution
3–19C What is the physical significance of hfg? Can it be obtained from a knowledge of hf and hg? How? Get 3.19 exercise solution
3–20C Is it true that it takes more energy to vaporize 1 kg of saturated liquid water at 100°C than it would at 120°C? Get 3.20 exercise solution
3–21C What is quality? Does it have any meaning in the superheated vapor region? Get 3.21 exercise solution
3–22C Which process requires more energy: completely vaporizing 1 kg of saturated liquid water at 1 atm pressure or completely vaporizing 1 kg of saturated liquid water at 8 atm pressure? Get 3.22 exercise solution
3–23C Does hfg change with pressure? How? Get 3.23 exercise solution
3–24C Can quality be expressed as the ratio of the volume occupied by the vapor phase to the total volume? Explain. Get 3.24 exercise solution
3–25C In the absence of compressed liquid tables, how is the specific volume of a compressed liquid at a given P and T determined? Get 3.25 exercise solution
3–26 Complete this table for H2O:

Get 3.26 exercise solution
3–27 Reconsider Prob. 3–26. Using EES (or other) software, determine the missing properties of water. Repeat the solution for refrigerant-134a, refrigerant22, and ammonia. Get 3.27 exercise solution
3–28E Complete this table for H2O:

Get 3.28 exercise solution
3–29E Reconsider Prob. 3–28E. Using EES (or other) software, determine the missing properties of water. Repeat the solution for refrigerant-134a, refrigerant22, and ammonia. Get 3.29 exercise solution
3–30 Complete this table for H2O:

Get 3.30 exercise solution
3–31 Complete this table for refrigerant-134a:

Get 3.31 exercise solution
3–32 Complete this table for refrigerant-134a:

Get 3.32 exercise solution
3–33E Complete this table for refrigerant-134a:

Get 3.33 exercise solution
3–34 Complete this table for H2O:

Get 3.34 exercise solution
3–35 Complete this table for H2O:
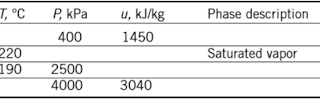
Get 3.35 exercise solution
3–36 A 1.8-m3 rigid tank contains steam at 220°C. Onethird of the volume is in the liquid phase and the rest is in the vapor form. Determine (a) the pressure of the steam, (b) the quality of the saturated mixture, and (c) the density of the mixture.

Get 3.36 exercise solution
3–37 A piston–cylinder device contains 0.85 kg of refrigerant134a at 10°C. The piston that is free to move has a mass of 12 kg and a diameter of 25 cm. The local atmospheric pressure is 88 kPa. Now, heat is transferred to refrigerant-134a until the temperature is 15°C. Determine (a) the final pressure, (b) the change in the volume of the cylinder, and (c) the change in the enthalpy of the refrigerant-134a.

Get 3.37 exercise solution
3–38E The temperature in a pressure cooker during cooking at sea level is measured to be 250°F. Determine the absolute pressure inside the cooker in psia and in atm. Would you modify your answer if the place were at a higher elevation? Get 3.38 exercise solution
3–39E The atmospheric pressure at a location is usually specified at standard conditions, but it changes with the weather conditions. As the weather forecasters frequently state, the atmospheric pressure drops during stormy weather and it rises during clear and sunny days. If the pressure difference between the two extreme conditions is given to be 0.3 in of mercury, determine how much the boiling temperatures of water will vary as the weather changes from one extreme to the other. Get 3.39 exercise solution
3–40 A person cooks a meal in a 30-cm-diameter pot that is covered with a well-fitting lid and lets the food cool to the room temperature of 20°C. The total mass of the food and the pot is 8 kg. Now the person tries to open the pan by lifting the lid up. Assuming no air has leaked into the pan during cooling, determine if the lid will open or the pan will move up together with the lid. Get 3.40 exercise solution
3–41 Water is to be boiled at sea level in a 30-cm-diameter stainless steel pan placed on top of a 3–kW electric burner. If 60 percent of the heat generated by the burner is transferred to the water during boiling, determine the rate of evaporation of water. Get 3.41 exercise solution
3–42 Repeat Prob. 3–41 for a location at an elevation of 1500 m where the atmospheric pressure is 84.5 kPa and thus the boiling temperature of water is 95°C. Get 3.42 exercise solution
3–43 Water is boiled at 1 atm pressure in a 25-cm-internaldiameter stainless steel pan on an electric range. If it is observed that the water level in the pan drops by 10 cm in 45 min, determine the rate of heat transfer to the pan. Get 3.43 exercise solution
3–44 Repeat Prob. 3–43 for a location at 2000-m elevation where the standard atmospheric pressure is 79.5 kPa. Get 3.44 exercise solution
3–45 Saturated steam coming off the turbine of a steam power plant at 30°C condenses on the outside of a 3-cmouter-diameter, 35-m-long tube at a rate of 45 kg/h. Determine the rate of heat transfer from the steam to the cooling water flowing through the pipe. Get 3.45 exercise solution
3–46 The average atmospheric pressure in Denver (elevation = 1610 m) is 83.4 kPa. Determine the temperature at which water in an uncovered pan boils in Denver. Get 3.46 exercise solution
3–47 Water in a 5-cm-deep pan is observed to boil at 98°C. At what temperature will the water in a 40-cm-deep pan boil? Assume both pans are full of water. Get 3.47 exercise solution
3–48 A cooking pan whose inner diameter is 20 cm is filled with water and covered with a 4-kg lid. If the local atmospheric pressure is 101 kPa, determine the temperature at which the water starts boiling when it is heated. Get 3.48 exercise solution
3–49 Reconsider Prob. 3–48. Using EES (or other) software, investigate the effect of the mass of the lid on the boiling temperature of water in the pan. Let the mass vary from 1 kg to 10 kg. Plot the boiling temperature against the mass of the lid, and discuss the results. Get 3.49 exercise solution
3–50 Water is being heated in a vertical piston–cylinder device. The piston has a mass of 20 kg and a cross-sectional area of 100 cm2. If the local atmospheric pressure is 100 kPa, determine the temperature at which the water starts boiling. Get 3.50 exercise solution
3–51 A rigid tank with a volume of 2.5 m3 contains 15 kg of saturated liquid–vapor mixture of water at 75°C. Now the water is slowly heated. Determine the temperature at which the liquid in the tank is completely vaporized. Also, show the process on a T-v diagram with respect to saturation lines. Get 3.51 exercise solution
3–52 A rigid vessel contains 2 kg of refrigerant-134a at 800 kPa and 120°C. Determine the volume of the vessel and the total internal energy. Get 3.52 exercise solution
3–53E A 5-ft3 rigid tank contains 5 lbm of water at 20 psia. Determine (a) the temperature, (b) the total enthalpy, and (c) the mass of each phase of water. Get 3.53 exercise solution
3–54 A 0.5-m3 vessel contains 10 kg of refrigerant-134a at -20°C. Determine (a) the pressure, (b) the total internal energy, and (c) the volume occupied by the liquid phase. Get 3.54 exercise solution
3–55 A piston–cylinder device contains 0.1 m3 of liquid water and 0.9 m3 of water vapor in equilibrium at 800 kPa. Heat is transferred at constant pressure until the temperature reaches 350°C. (a) What is the initial temperature of the water? (b) Determine the total mass of the water. (c) Calculate the final volume. (d) Show the process on a P-v diagram with respect to saturation lines. Get 3.55 exercise solution
3–56 Reconsider Prob. 3–55. Using EES (or other) software, investigate the effect of pressure on the total mass of water in the tank. Let the pressure vary from 0.1 MPa to 1 MPa. Plot the total mass of water against pressure, and discuss the results. Also, show the process in Prob. 3–55 on a P-v diagram using the property plot feature of EES. Get 3.56 exercise solution
3–57E Superheated water vapor at 180 psia and 500°F is allowed to cool at constant volume until the temperature drops to 250°F. At the final state, determine (a) the pressure, (b) the quality, and (c) the enthalpy. Also, show the process on a T-v diagram with respect to saturation lines. Get 3.57 exercise solution
3–58E Reconsider Prob. 3–57E. Using EES (or other) software, investigate the effect of initial pressure on the quality of water at the final state. Let the pressure vary from 100 psi to 300 psi. Plot the quality against initial pressure, and discuss the results. Also, show the process in Prob. 3–57E on a T-v diagram using the property plot feature of EES. Get 3.58 exercise solution
3–59 A piston–cylinder device initially contains 50 L of liquid water at 40°C and 200 kPa. Heat is transferred to the water at constant pressure until the entire liquid is vaporized. (a) What is the mass of the water? (b) What is the final temperature? (c) Determine the total enthalpy change. (d) Show the process on a T-v diagram with respect to saturation lines. Answers: (a) 49.61 kg, (b) 120.21°C, (c) 125,943 kJ Get 3.59 exercise solution
3–60 A 0.3-m3 rigid vessel initially contains saturated liquid– vapor mixture of water at 150°C. The water is now heated until it reaches the critical state. Determine the mass of the liquid water and the volume occupied by the liquid at the initial state. Get 3.60 exercise solution
3–61 Determine the specific volume, internal energy, and enthalpy of compressed liquid water at 100°C and 15 MPa using the saturated liquid approximation. Compare these values to the ones obtained from the compressed liquid tables. Get 3.61 exercise solution
3–62 Reconsider Prob. 3–61. Using EES (or other) software, determine the indicated properties of compressed liquid, and compare them to those obtained using the saturated liquid approximation. Get 3.62 exercise solution
3–63E A 15-ft3 rigid tank contains a saturated mixture of refrigerant-134a at 50 psia. If the saturated liquid occupies 20 percent of the volume, determine the quality and the total mass of the refrigerant in the tank. Get 3.63 exercise solution
3–64 A piston–cylinder device contains 0.8 kg of steam at 300°C and 1 MPa. Steam is cooled at constant pressure until one-half of the mass condenses. (a) Show the process on a T-v diagram. (b) Find the final temperature. (c) Determine the volume change. Get 3.64 exercise solution
3–65 A rigid tank contains water vapor at 250°C and an unknown pressure. When the tank is cooled to 150°C, the vapor starts condensing. Estimate the initial pressure in the tank. Get 3.65 exercise solution
3–66 Water is boiled in a pan covered with a poorly fitting lid at a specified location. Heat is supplied to the pan by a 2-kW resistance heater. The amount of water in the pan is observed to decrease by 1.19 kg in 30 minutes. If it is estimated that 75 percent of electricity consumed by the heater is transferred to the water as heat, determine the local atmospheric pressure in that location. Get 3.66 exercise solution
3–67 A rigid tank initially contains 1.4-kg saturated liquid water at 200°C. At this state, 25 percent of the volume is occupied by water and the rest by air. Now heat is supplied to the water until the tank contains saturated vapor only. Determine (a) the volume of the tank, (b) the final temperature and pressure, and (c) the internal energy change of the water. Get 3.67 exercise solution
3–68 A piston–cylinder device initially contains steam at 3.5 MPa, superheated by 5°C. Now, steam loses heat to the surroundings and the piston moves down hitting a set of stops at which point the cylinder contains saturated liquid water. The cooling continues until the cylinder contains water at 200°C. Determine (a) the initial temperature, (b) the enthalpy change per unit mass of the steam by the time the piston first hits the stops, and (c) the final pressure and the quality (if mixture). Get 3.68 exercise solution
3–69C Propane and methane are commonly used for heating in winter, and the leakage of these fuels, even for short periods, poses a fire danger for homes. Which gas leakage do you think poses a greater risk for fire? Explain. Get 3.69 exercise solution
3–70C Under what conditions is the ideal-gas assumption suitable for real gases? Get 3.70 exercise solution
3–71C What is the difference between R and Ru? How are these two related? Get 3.71 exercise solution
3–72C What is the difference between mass and molar mass? How are these two related? Get 3.72 exercise solution
3–73 A spherical balloon with a diameter of 6 m is filled with helium at 20°C and 200 kPa. Determine the mole number and the mass of the helium in the balloon. Get 3.73 exercise solution
3–74 Reconsider Prob. 3–73. Using EES (or other) software, investigate the effect of the balloon diameter on the mass of helium contained in the balloon for the pressures of (a) 100 kPa and (b) 200 kPa. Let the diameter vary from 5 m to 15 m. Plot the mass of helium against the diameter for both cases. Get 3.74 exercise solution
3–75 The pressure in an automobile tire depends on the temperature of the air in the tire. When the air temperature is 25°C, the pressure gage reads 210 kPa. If the volume of the tire is 0.025 m3, determine the pressure rise in the tire when the air temperature in the tire rises to 50°C. Also, determine the amount of air that must be bled off to restore pressure to its original value at this temperature. Assume the atmospheric pressure is 100 kPa. Get 3.75 exercise solution
3–76E The air in an automobile tire with a volume of 0.53 ft3 is at 90°F and 20 psig. Determine the amount of air that must be added to raise the pressure to the recommended value of 30 psig. Assume the atmospheric pressure to be 14.6 psia and the temperature and the volume to remain constant. Get 3.76 exercise solution
3–77 The pressure gage on a 2.5-m3 oxygen tank reads 500 kPa. Determine the amount of oxygen in the tank if the temperature is 28°C and the atmospheric pressure is 97 kPa. Get 3.77 exercise solution
3–78E A rigid tank contains 20 lbm of air at 20 psia and 70°F. More air is added to the tank until the pressure and temperature rise to 35 psia and 90°F, respectively. Determine the amount of air added to the tank. Get 3.78 exercise solution
3–79 A 400-L rigid tank contains 5 kg of air at 25°C. Determine the reading on the pressure gage if the atmospheric pressure is 97 kPa. Get 3.79 exercise solution
3–80 A 1-m3 tank containing air at 25°C and 500 kPa is connected through a valve to another tank containing 5 kg of air at 35°C and 200 kPa. Now the valve is opened, and the entire system is allowed to reach thermal equilibrium with the surroundings, which are at 20°C. Determine the volume of the second tank and the final equilibrium pressure of air Get 3.80 exercise solution
3–81C What is the physical significance of the compressibility factor Z? Get 3.81 exercise solution
3–82C What is the principle of corresponding states? Get 3.82 exercise solution
3–83C How are the reduced pressure and reduced temperature defined? Get 3.83 exercise solution
3–84 Determine the specific volume of superheated water vapor at 10 MPa and 400°C, using (a) the ideal-gas equation, (b) the generalized compressibility chart, and (c) the steam tables. Also determine the error involved in the first two cases Get 3.84 exercise solution
3–85 Reconsider Prob. 3–84. Solve the problem using the generalized compressibility factor feature of the EES software. Again using EES, compare the specific volume of water for the three cases at 10 MPa over the temperature range of 325 to 600°C in 25°C intervals. Plot the percent error involved in the ideal-gas approximation against temperature, and discuss the results. Get 3.85 exercise solution
3–86 Determine the specific volume of refrigerant-134a vapor at 0.9 MPa and 70°C based on (a) the ideal-gas equation, (b) the generalized compressibility chart, and (c) data from tables. Also, determine the error involved in the first two cases. Get 3.86 exercise solution
3–87 Determine the specific volume of nitrogen gas at 10 MPa and 150 K based on (a) the ideal-gas equation and (b) the generalized compressibility chart. Compare these results with the experimental value of 0.002388 m3/kg, and determine the error involved in each case. Get 3.87 exercise solution
3–88 Determine the specific volume of superheated water vapor at 3.5 MPa and 450°C based on (a) the ideal-gas equation, (b) the generalized compressibility chart, and (c) the steam tables. Determine the error involved in the first two cases. Get 3.88 exercise solution
3–89E Refrigerant-134a at 400 psia has a specific volume of 0.13853 ft3/lbm. Determine the temperature of the refrigerant based on (a) the ideal-gas equation, (b) the generalized compressibility chart, and (c) the refrigerant tables. Get 3.89 exercise solution
3–90 A 0.016773-m3 tank contains 1 kg of refrigerant-134a at 110°C. Determine the pressure of the refrigerant, using (a) the ideal-gas equation, (b) the generalized compressibility chart, and (c) the refrigerant tables. Get 3.90 exercise solution
3–91 Somebody claims that oxygen gas at 160 K and 3 MPa can be treated as an ideal gas with an error of less than 10 percent. Is this claim valid? Get 3.91 exercise solution
3–92 What is the percentage of error involved in treating carbon dioxide at 3 MPa and 10°C as an ideal gas? Get 3.92 exercise solution
3–93 What is the percentage of error involved in treating carbon dioxide at 7 MPa and 380 K as an ideal gas? Get 3.93 exercise solution
3–94 Carbon dioxide gas enters a pipe at 3 MPa and 500 K at a rate of 2 kg/s. CO2 is cooled at constant pressure as it flows in the pipe and the temperature CO2 drops to 450 K at the exit. Determine the volume flow rate and the density of carbon dioxide at the inlet and the volume flow rate at the exit of the pipe using (a) the ideal-gas equation and (b) the generalized compressibility chart. Also, determine (c) the error involved in each case.
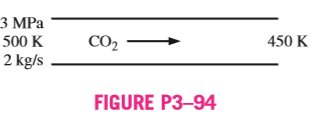
Get 3.94 exercise solution
3–95C What is the physical significance of the two constants that appear in the van der Waals equation of state? On what basis are they determined? Get 3.95 exercise solution
3–96 A 3.27-m3 tank contains 100 kg of nitrogen at 175 K. Determine the pressure in the tank, using (a) the ideal-gas equation, (b) the van der Waals equation, and (c) the BeattieBridgeman equation. Compare your results with the actual value of 1505 kPa. Get 3.96 exercise solution
3–97 A 1-m3 tank contains 2.841 kg of steam at 0.6 MPa. Determine the temperature of the steam, using (a) the idealgas equation, (b) the van der Waals equation, and (c) the steam tables. Get 3.97 exercise solution
3–98 Reconsider Prob. 3–97. Solve the problem using EES (or other) software. Again using the EES, compare the temperature of water for the three cases at constant specific volume over the pressure range of 0.1 MPa to 1 MPa in 0.1 MPa increments. Plot the percent error involved in the ideal-gas approximation against pressure, and discuss the results. Get 3.98 exercise solution
3–99E Refrigerant-134a at 100 psia has a specific volume of 0.54022 ft3/lbm. Determine the temperature of the refrigerant based on (a) the ideal-gas equation, (b) the van der Waals equation, and (c) the refrigerant tables. Get 3.99 exercise solution
3–100 Nitrogen at 150 K has a specific volume of 0.041884 m3/kg. Determine the pressure of the nitrogen, using (a) the ideal-gas equation and (b) the BeattieBridgeman equation. Compare your results to the experimental value of 1000 kPa. Answers: (a) 1063 kPa, (b) 1000.4 kPa Get 3.100 exercise solution
3–101 Reconsider Prob. 3–100. Using EES (or other) software, compare the pressure results of the ideal-gas and Beattie-Bridgeman equations with nitrogen data supplied by EES. Plot temperature versus specific volume for a pressure of 1000 kPa with respect to the saturated liquid and saturated vapor lines of nitrogen over the range of 110 K < T < 150 K. Get 3.101 exercise solution
3–102 Consider a glass of water in a room that is at 20°C and 60 percent relative humidity. If the water temperature is 15°C, determine the vapor pressure (a) at the free surface of the water and (b) at a location in the room far from the glass. Get 3.102 exercise solution
3–103 During a hot summer day at the beach when the air temperature is 30°C, someone claims the vapor pressure in the air to be 5.2 kPa. Is this claim reasonable? Get 3.103 exercise solution
3–104 On a certain day, the temperature and relative humidity of air over a large swimming pool are measured to be 20°C and 40 percent, respectively. Determine the water temperature of the pool when phase equilibrium conditions are established between the water in the pool and the vapor in the air. Get 3.104 exercise solution
3–105 Consider two rooms that are identical except that one is maintained at 30°C and 40 percent relative humidity while the other is maintained at 20°C and 70 percent relative humidity. Noting that the amount of moisture is proportional to the vapor pressure, determine which room contains more moisture. Get 3.105 exercise solution
3–106E A thermos bottle is half-filled with water and is left open to the atmospheric air at 70°F and 35 percent relative humidity. If heat transfer to the water through the thermos walls and the free surface is negligible, determine the temperature of water when phase equilibrium is established. Get 3.106 exercise solution
3–107 During a hot summer day when the air temperature is 35°C and the relative humidity is 70 percent, you buy a supposedly “cold” canned drink from a store. The store owner claims that the temperature of the drink is below 10°C. Yet the drink does not feel so cold and you are skeptical since you notice no condensation forming outside the can. Can the store owner be telling the truth? Get 3.107 exercise solution
3–108 The combustion in a gasoline engine may be approximated by a constant volume heat addition process. There exists the air–fuel mixture in the cylinder before the combustion and the combustion gases after it, and both may be approximated as air, an ideal gas. In a gasoline engine, the cylinder conditions are 1.8 MPa and 450°C before the combustion and 1300°C after it. Determine the pressure at the end of the combustion process. Get 3.108 exercise solution
3–109 A rigid tank contains an ideal gas at 300 kPa and 600 K. Now half of the gas is withdrawn from the tank and the gas is found at 100 kPa at the end of the process. Determine (a) the final temperature of the gas and (b) the final pressure if no mass was withdrawn from the tank and the same final temperature was reached at the end of the process. Get 3.109 exercise solution
3–110 Carbon-dioxide gas at 3 MPa and 500 K flows steadily in a pipe at a rate of 0.4 kmol/s. Determine (a) the volume and mass flow rates and the density of carbon dioxide at this state. If CO2 is cooled at constant pressure as it flows in the pipe so that the temperature of CO2 drops to 450 K at the exit of the pipe, determine (b) the volume flow rate at the exit of the pipe. Get 3.110 exercise solution
3–111 A piston–cylinder device initially contains 0.2 kg of steam at 200 kPa and 300°C. Now, the steam is cooled at constant pressure until it is at 150°C. Determine the volume change of the cylinder during this process using the compressibility factor and compare the result to the actual value.

Get 3.111 exercise solution
3–112 Combustion in a diesel engine may be modeled as a constant-pressure heat addition process with air in the cylinder before and after combustion. Consider a diesel engine with cylinder conditions of 950 K and 75 cm3 before combustion, and 150 cm3 after it. The engine operates with an air–fuel ratio of 22 kg air/kg fuel (the mass of the air divided by the mass of the fuel). Determine the temperature after the combustion process. Get 3.112 exercise solution
3–113 On the property diagrams indicated below, sketch (not to scale) with respect to the saturated liquid and saturated vapor lines and label the following processes and states for steam. Use arrows to indicate the direction of the process, and label the initial and final states: (a) On the P-v diagram sketch the constant temperature process through the state P = 300 kPa, v = 0.525 m3/kg as pressure changes from P1 = 200 kPa to P2 = 400 kPa. Place the value of the temperature on the process curve on the P-v diagram. (b) On the T-v diagram sketch the constant specific volume process through the state T = 120°C, v = 0.7163 m3/kg from P1 = 100 kPa to P2 = 300 kPa. For this data set place the temperature values at states 1 and 2 on its axis. Place the value of the specific volume on its axis. Get 3.113 exercise solution
3–114 The gage pressure of an automobile tire is measured to be 200 kPa before a trip and 220 kPa after the trip at a location where the atmospheric pressure is 90 kPa. Assuming the volume of the tire remains constant at 0.035 m3, determine the percent increase in the absolute temperature of the air in the tire. Get 3.114 exercise solution
3–115 Although balloons have been around since 1783 when the first balloon took to the skies in France, a real breakthrough in ballooning occurred in 1960 with the design of the modern hot-air balloon fueled by inexpensive propane and constructed of lightweight nylon fabric. Over the years, ballooning has become a sport and a hobby for many people around the world. Unlike balloons filled with the light helium gas, hot-air balloons are open to the atmosphere. Therefore, the pressure in the balloon is always the same as the local atmospheric pressure, and the balloon is never in danger of exploding. Hot-air balloons range from about 15 to 25 m in diameter. The air in the balloon cavity is heated by a propane burner located at the top of the passenger cage. The flames from the burner that shoot into the balloon heat the air in the balloon cavity, raising the air temperature at the top of the balloon from 65°C to over 120°C. The air temperature is maintained at the desired levels by periodically firing the propane burner. The buoyancy force that pushes the balloon upward is proportional to the density of the cooler air outside the balloon and the volume of the balloon, and can be expressed as FB = pcool air gVballoon where g is the gravitational acceleration. When air resistance is negligible, the buoyancy force is opposed by (1) the weight of the hot air in the balloon, (2) the weight of the cage, the ropes, and the balloon material, and (3) the weight of the people and other load in the cage. The operator of the balloon can control the height and the vertical motion of the balloon by firing the burner or by letting some hot air in the balloon escape, to be replaced by cooler air. The forward motion of the balloon is provided by the winds. Consider a 20-m-diameter hot-air balloon that, together with its cage, has a mass of 80 kg when empty. This balloon is hanging still in the air at a location where the atmospheric pressure and temperature are 90 kPa and 15°C, respectively, while carrying three 65-kg people. Determine the average temperature of the air in the balloon. What would your response be if the atmospheric air temperature were 30°C? Get 3.115 exercise solution
3–116 Reconsider Prob. 3–115. Using EES (or other) software, investigate the effect of the environment temperature on the average air temperature in the balloon when the balloon is suspended in the air. Assume the environment temperature varies from 10 to 30°C. Plot the average air temperature in the balloon versus the environment temperature, and discuss the results. Investigate how the number of people carried affects the temperature of the air in the balloon. Get 3.116 exercise solution
3–117 Consider an 18-m-diameter hot-air balloon that, together with its cage, has a mass of 120 kg when empty. The air in the balloon, which is now carrying two 70-kg people, is heated by propane burners at a location where the atmospheric pressure and temperature are 93 kPa and 12°C, respectively. Determine the average temperature of the air in the balloon when the balloon first starts rising. What would your response be if the atmospheric air temperature were 25°C? Get 3.117 exercise solution
3–118E Water in a pressure cooker is observed to boil at 260°F. What is the absolute pressure in the pressure cooker, in psia? Get 3.118 exercise solution
3–119 A rigid tank with a volume of 0.117 m3 contains 1 kg of refrigerant-134a vapor at 240 kPa. The refrigerant is now allowed to cool. Determine the pressure when the refrigerant first starts condensing. Also, show the process on a P-v diagram with respect to saturation lines. Get 3.119 exercise solution
3–120 A 4-L rigid tank contains 2 kg of saturated liquid–vapor mixture of water at 50°C. The water is now slowly heated until it exists in a single phase. At the final state, will the water be in the liquid phase or the vapor phase? What would your answer be if the volume of the tank were 400 L instead of 4 L? Get 3.120 exercise solution
3–121 A 10-kg mass of superheated refrigerant-134a at 1.2 MPa and 70°C is cooled at constant pressure until it exists as a compressed liquid at 20°C. (a) Show the process on a T-v diagram with respect to saturation lines. (b) Determine the change in volume. (c) Find the change in total internal energy. Get 3.121 exercise solution
3–122 A 0.5-m3 rigid tank containing hydrogen at 20°C and 600 kPa is connected by a valve to another 0.5-m3 rigid tank that holds hydrogen at 30°C and 150 kPa. Now the valve is opened and the system is allowed to reach thermal equilibrium with the surroundings, which are at 15°C. Determine the final pressure in the tank.

Get 3.122 exercise solution
3–123 Reconsider Prob. 3–122. Using EES (or other) software, investigate the effect of the surroundings temperature on the final equilibrium pressure in the tanks. Assume the surroundings temperature to vary from -10 to 30°C. Plot the final pressure in the tanks versus the surroundings temperature, and discuss the results. Get 3.123 exercise solution
3–124 A 20-m3 tank contains nitrogen at 23°C and 600 kPa. Some nitrogen is allowed to escape until the pressure in the tank drops to 400 kPa. If the temperature at this point is 20°C, determine the amount of nitrogen that has escaped Get 3.124 exercise solution
3–125 Steam at 400°C has a specific volume of 0.02 m3/kg. Determine the pressure of the steam based on (a) the idealgas equation, (b) the generalized compressibility chart, and (c) the steam tables. Get 3.125 exercise solution
3–126 A tank whose volume is unknown is divided into two parts by a partition. One side of the tank contains 0.01 m3 of refrigerant-134a that is a saturated liquid at 0.8 MPa, while the other side is evacuated. The partition is now removed, and the refrigerant fills the entire tank. If the final state of the refrigerant is 20°C and 400 kPa, determine the volume of the tank. Get 3.126 exercise solution
3–127 Reconsider Prob. 3–126. Using EES (or other) software, investigate the effect of the initial pressure of refrigerant-134a on the volume of the tank. Let the initial pressure vary from 0.5 to 1.5 MPa. Plot the volume of the tank versus the initial pressure, and discuss the results. Get 3.127 exercise solution
3–128 Liquid propane is commonly used as a fuel for heating homes, powering vehicles such as forklifts, and filling portable picnic tanks. Consider a propane tank that initially contains 5 L of liquid propane at the environment temperature of 20°C. If a hole develops in the connecting tube of a propane tank and the propane starts to leak out, determine the temperature of propane when the pressure in the tank drops to 1 atm. Also, determine the total amount of heat transfer from the environment to the tank to vaporize the entire propane in the tank.

Get 3.128 exercise solution
3–129 Repeat Prob. 3–128 for isobutane. Get 3.129 exercise solution
3–130 A tank contains helium at 100°C and 10 kPa gage. The helium is heated in a process by heat transfer from the surroundings such that the helium reaches a final equilibrium state at 300°C. Determine the final gage pressure of the helium. Assume atmospheric pressure is 100 kPa. Get 3.130 exercise solution
3–13l A tank contains argon at 600°C and 200 kPa gage. The argon is cooled in a process by heat transfer to the surroundings such that the argon reaches a final equilibrium state at 300°C. Determine the final gage pressure of the argon. Assume atmospheric pressure is 100 kPa. Get 3.131 exercise solution
3–132 Complete the blank cells in the following table of properties of steam. In the last column describe the condition of steam as compressed liquid, saturated mixture, superheated vapor, or insufficient information; and, if applicable, give the quality.

Get 3.132 exercise solution
3–133 Complete the blank cells in the following table of properties of refrigerant-134a. In the last column describe the condition of refrigerant-134a as compressed liquid, saturated mixture, superheated vapor, or insufficient information; and, if applicable, give the quality.
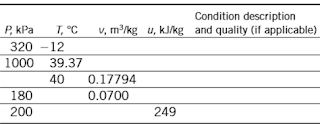
Get 3.133 exercise solution
3–134 On the property diagrams indicated below, sketch (not to scale) with respect to the saturated liquid and saturated vapor lines and label the following processes and states for refrigerant-134a. Use arrows to indicate the direction of the process, and label the initial and final states: (a) On the P-v diagram sketch the constant temperature process through the state P = 280 kPa, v = 0.06 m3/kg as pressure changes from P1 = 400 kPa to P2 = 200 kPa. Place the value of the temperature on the process curve on the P-v diagram. (b) On the T-v diagram sketch the constant specific volume process through the state T = 20°C, v = 0.02 m3/kg from P1 1200 kPa to P2 = 300 kPa. For this data set place the temperature values at states 1 and 2 on its axis. Place the value of the specific volume on its axis. Get 3.134 exercise solution
3–135 A rigid tank contains 6 kg of an ideal gas at 3 atm and 40°C. Now a valve is opened, and half of mass of the gas is allowed to escape. If the final pressure in the tank is 2.2 atm, the final temperature in the tank is (a) 186°C (b) 59° (c) -43°C (d) 20°C (e) 230°C Get 3.135 exercise solution
3–136 The pressure of an automobile tire is measured to be 190 kPa (gage) before a trip and 215 kPa (gage) after the trip at a location where the atmospheric pressure is 95 kPa. If the temperature of air in the tire before the trip is 25°C, the air temperature after the trip is (a) 51.1°C (b) 64.2°C (c) 27.2°C (d) 28.3°C (e) 25.0°C Get 3.136 exercise solution
3–137 A 300-m3 rigid tank is filled with saturated liquid– vapor mixture of water at 200 kPa. If 25 percent of the mass is liquid and 75 percent of the mass is vapor, the total mass in the tank is (a) 451 kg (b) 556 kg (c) 300 kg (d) 331 kg (e) 195 kg Get 3.137 exercise solution
3–138 Water is boiled at 1 atm pressure in a coffee maker equipped with an immersion-type electric heating element. The coffee maker initially contains 1 kg of water. Once boiling started, it is observed that half of the water in the coffee maker evaporated in 18 minutes. If the heat loss from the coffee maker is negligible, the power rating of the heating element is (a) 0.90 kW (d) 1.05 kW (b) 1.52 kW (e) 1.24 kW (c) 2.09 kW Get 3.138 exercise solution
3–139 A 1-m3 rigid tank contains 10 kg of water (in any phase or phases) at 160°C. The pressure in the tank is (a) 738 kPa (d) 2000 MPa (b) 618 kPa (e) 1618 kPa (c) 370 kPa Get 3.139 exercise solution
3–140 Water is boiling at 1 atm pressure in a stainless steel pan on an electric range. It is observed that 2 kg of liquid water evaporates in 30 min. The rate of heat transfer to the water is (a) 2.51 kW (d) 0.47 kW (b) 2.32 kW (e) 3.12 kW (c) 2.97 kW Get 3.140 exercise solution
3–141 Water is boiled in a pan on a stove at sea level. During 10 min of boiling, it is observed that 200 g of water has evaporated. Then the rate of heat transfer to the water is (a) 0.84 kJ/min (d) 53.5 kJ/min (b) 45.1 kJ/min (e) 225.7 kJ/min (c) 41.8 kJ/min Get 3.141 exercise solution
3–142 A 3-m3 rigid vessel contains steam at 10 MPa and 500°C. The mass of the steam is (a) 3.0 kg (b) 19 kg (c) 84 kg (d) 91 kg (e) 130 kg Get 3.142 exercise solution
3–143 Consider a sealed can that is filled with refrigerant134a. The contents of the can are at the room temperature of 25°C. Now a leak develops, and the pressure in the can drops to the local atmospheric pressure of 90 kPa. The temperature of the refrigerant in the can is expected to drop to (rounded to the nearest integer) (a) 0°C (b) -29°C (c) -16°C (d) 5°C (e) 25°C Get 3.143 exercise solution
3–144 A solid normally absorbs heat as it melts, but there is a known exception at temperatures close to absolute zero. Find out which solid it is and give a physical explanation for it. Get 3.144 exercise solution