7–2C Does a cycle for which phidQ > 0 violate the Clausius inequality? Why? Get 7.2 exercise solution
7–3C Is a quantity whose cyclic integral is zero necessarily a property? Get 7.3 exercise solution
7–4C Does the cyclic integral of heat have to be zero (i.e., does a system have to reject as much heat as it receives to complete a cycle)? Explain. Get 7.4 exercise solution
7–5C Does the cyclic integral of work have to be zero (i.e., does a system have to produce as much work as it consumes to complete a cycle)? Explain. Get 7.5 exercise solution
7–6C A system undergoes a process between two fixed states first in a reversible manner and then in an irreversible manner. For which case is the entropy change greater? Why? Get 7.6 exercise solution
7–7C Is the value of the integral f 1 2 dQ/T the same for all processes between states 1 and 2? Explain. Get 7.7 exercise solution
7–8C Is the value of the integral f 1 2 dQ/T the same for all reversible processes between states 1 and 2? Why? Get 7.8 exercise solution
7–9C To determine the entropy change for an irreversible process between states 1 and 2, should the integral f 1 2 dQ/T be performed along the actual process path or an imaginary reversible path? Explain. Get 7.9 exercise solution
7–10C Is an isothermal process necessarily internally reversible? Explain your answer with an example. Get 7.10 exercise solution
7–11C How do the values of the integral f 1 2 dQ/T compare for a reversible and irreversible process between the same end states? Get 7.11 exercise solution
7–12C The entropy of a hot baked potato decreases as it cools. Is this a violation of the increase of entropy principle? Explain. Get 7.12 exercise solution
7–13C Is it possible to create entropy? Is it possible to destroy it? Get 7.13 exercise solution
7–14C A piston–cylinder device contains helium gas. During a reversible, isothermal process, the entropy of the helium will (never, sometimes, always) increase. Get 7.14 exercise solution
7–15C A piston–cylinder device contains nitrogen gas. During a reversible, adiabatic process, the entropy of the nitrogen will (never, sometimes, always) increase. Get 7.15 exercise solution
7–16C A piston–cylinder device contains superheated steam. During an actual adiabatic process, the entropy of the steam will (never, sometimes, always) increase. Get 7.16 exercise solution
7–17C The entropy of steam will (increase, decrease, remain the same) as it flows through an actual adiabatic turbine. Get 7.17 exercise solution
7–18C The entropy of the working fluid of the ideal Carnot cycle (increases, decreases, remains the same) during the isothermal heat addition process. Get 7.18 exercise solution
7–19C The entropy of the working fluid of the ideal Carnot cycle (increases, decreases, remains the same) during the isothermal heat rejection process. Get 7.19 exercise solution
7–20C During a heat transfer process, the entropy of a system (always, sometimes, never) increases. Get 7.20 exercise solution
7–21C Is it possible for the entropy change of a closed system to be zero during an irreversible process? Explain. Get 7.21 exercise solution
7–22C What three different mechanisms can cause the entropy of a control volume to change? Get 7.22 exercise solution
7–23C Steam is accelerated as it flows through an actual adiabatic nozzle. The entropy of the steam at the nozzle exit will be (greater than, equal to, less than) the entropy at the nozzle inlet. Get 7.23 exercise solution
7–24 A rigid tank contains an ideal gas at 40°C that is being stirred by a paddle wheel. The paddle wheel does 200 kJ of work on the ideal gas. It is observed that the temperature of the ideal gas remains constant during this process as a result of heat transfer between the system and the surroundings at 30°C. Determine the entropy change of the ideal gas. Get 7.24 exercise solution
7–25 Air is compressed by a 12-kW compressor from P1 to P2. The air temperature is maintained constant at 25°C during this process as a result of heat transfer to the surrounding medium at 10°C. Determine the rate of entropy change of the air. State the assumptions made in solving this problem. Get 7.25 exercise solution
7–26 During the isothermal heat addition process of a Carnot cycle, 900 kJ of heat is added to the working fluid from a source at 400°C. Determine (a) the entropy change of the working fluid, (b) the entropy change of the source, and (c) the total entropy change for the process. Get 7.26 exercise solution
7–27 Reconsider Prob. 7–26. Using EES (or other) software, study the effects of the varying heat added to the working fluid and the source temperature on the entropy change of the working fluid, the entropy change of the source, and the total entropy change for the process. Let the source temperature vary from 100 to 1000°C. Plot the entropy changes of the source and of the working fluid against the source temperature for heat transfer amounts of 500 kJ, 900 kJ, and 1300 kJ, and discuss the results. Get 7.27 exercise solution
7–28E During the isothermal heat rejection process of a Carnot cycle, the working fluid experiences an entropy change of -0.7 Btu/R. If the temperature of the heat sink is 95°F, determine (a) the amount of heat transfer, (b) the entropy change of the sink, and (c) the total entropy change for this process. Get 7.28 exercise solution
7–29 Refrigerant-134a enters the coils of the evaporator of a refrigeration system as a saturated liquid–vapor mixture at a pressure of 160 kPa. The refrigerant absorbs 180 kJ of heat from the cooled space, which is maintained at -5°C, and leaves as saturated vapor at the same pressure. Determine (a) the entropy change of the refrigerant, (b) the entropy change of the cooled space, and (c) the total entropy change for this process. Get 7.29 exercise solution
7–30C Is a process that is internally reversible and adiabatic necessarily isentropic? Explain. Get 7.30 exercise solution
7–31 The radiator of a steam heating system has a volume of 20 L and is filled with superheated water vapor at 200 kPa and 150°C. At this moment both the inlet and the exit valves to the radiator are closed. After a while the temperature of the steam drops to 40°C as a result of heat transfer to the room air. Determine the entropy change of the steam during this process. Get 7.31 exercise solution
7–32 A 0.5-m3 rigid tank contains refrigerant-134a initially at 200 kPa and 40 percent quality. Heat is transferred now to the refrigerant from a source at 35°C until the pressure rises to 400 kPa. Determine (a) the entropy change of the refrigerant, (b) the entropy change of the heat source, and (c) the total entropy change for this process. Get 7.32 exercise solution
7–33 Reconsider Prob. 7–32. Using EES (or other) software, investigate the effects of the source temperature and final pressure on the total entropy change for the process. Let the source temperature vary from 30 to 210°C, and the final pressure vary from 250 to 500 kPa. Plot the total entropy change for the process as a function of the source temperature for final pressures of 250 kPa, 400 kPa, and 500 kPa, and discuss the results. Get 7.33 exercise solution
7–34 A well-insulated rigid tank contains 2 kg of a saturated liquid–vapor mixture of water at 100 kPa. Initially, three-quarters of the mass is in the liquid phase. An electric resistance heater placed in the tank is now turned on and kept on until all the liquid in the tank is vaporized. Determine the entropy change of the steam during this process. Get 7.34 exercise solution
7–35 A rigid tank is divided into two equal parts by a partition. One part of the tank contains 1.5 kg of compressed liquid water at 300 kPa and 60°C while the other part is evacuated. The partition is now removed, and the water expands to fill the entire tank. Determine the entropy change of water during this process, if the final pressure in the tank is 15 kPa. Get 7.35 exercise solution
7–36 Reconsider Prob. 7–35. Using EES (or other) software, evaluate and plot the entropy generated as a function of surrounding temperature, and determine the values of the surrounding temperatures that are valid for this problem. Let the surrounding temperature vary from 0 to 100°C. Discuss your results. Get 7.36 exercise solution
7–37E A piston–cylinder device contains 2 lbm of refrigerant-134a at 120 psia and 100°F. The refrigerant is now cooled at constant pressure until it exists as a liquid at 50°F. Determine the entropy change of the refrigerant during this process. Get 7.37 exercise solution
7–38 An insulated piston–cylinder device contains 5 L of saturated liquid water at a constant pressure of 150 kPa. An electric resistance heater inside the cylinder is now turned on, and 2200 kJ of energy is transferred to the steam. Determine the entropy change of the water during this process. Get 7.38 exercise solution
7–39 An insulated piston–cylinder device contains 0.05 m3 of saturated refrigerant-134a vapor at 0.8-MPa pressure. The refrigerant is now allowed to expand in a reversible manner until the pressure drops to 0.4 MPa. Determine (a) the final temperature in the cylinder and (b) the work done by the refrigerant. Get 7.39 exercise solution
7–40 Reconsider Prob. 7–39. Using EES (or other) software, evaluate and plot the work done by the refrigerant as a function of final pressure as it varies from 0.8 to 0.4 MPa. Compare the work done for this process to one for which the temperature is constant over the same pressure range. Discuss your results. Get 7.40 exercise solution
7–41 Refrigerant-134a enters an adiabatic compressor as saturated vapor at 160 kPa at a rate of 2 m3/min and is compressed to a pressure of 900 kPa. Determine the minimum power that must be supplied to the compressor. Get 7.41 exercise solution
7–42E Steam enters an adiabatic turbine at 800 psia and 900°F and leaves at a pressure of 40 psia. Determine the maximum amount of work that can be delivered by this turbine. Get 7.42 exercise solution
7–43E Reconsider Prob. 7–42E. Using EES (or other) software, evaluate and plot the work done by the steam as a function of final pressure as it varies from 800 to 40 psia. Also investigate the effect of varying the turbine inlet temperature from the saturation temperature at 800 psia to 900°F on the turbine work. Get 7.43 exercise solution
7–44 A heavily insulated piston–cylinder device contains 0.05 m3 of steam at 300 kPa and 150°C. Steam is now compressed in a reversible manner to a pressure of 1 MPa. Determine the work done on the steam during this process. Get 7.44 exercise solution
7–45 Reconsider Prob. 7–44. Using EES (or other) software, evaluate and plot the work done on the steam as a function of final pressure as the pressure varies from 300 kPa to 1 MPa. Get 7.45 exercise solution
7–46 A piston–cylinder device contains 1.2 kg of saturated water vapor at 200°C. Heat is now transferred to steam, and steam expands reversibly and isothermally to a final pressure of 800 kPa. Determine the heat transferred and the work done during this process. Get 7.46 exercise solution
7–47 Reconsider Prob. 7–46. Using EES (or other) software, evaluate and plot the heat transferred to the steam and the work done as a function of final pressure as the pressure varies from the initial value to the final value of 800 kPa. Get 7.47 exercise solution
7–48 A piston–cylinder device contains 5 kg of steam at 100°C with a quality of 50 percent. This steam undergoes two processes as follows: 1-2 Heat is transferred to the steam in a reversible manner while the temperature is held constant until the steam exists as a saturated vapor. 2-3 The steam expands in an adiabatic, reversible process until the pressure is 15 kPa. (a) Sketch these processes with respect to the saturation lines on a single T-s diagram. (b) Determine the heat added to the steam in process 1-2, in kJ. (c) Determine the work done by the steam in process 2-3, in kJ. Get 7.48 exercise solution
7–49 A rigid tank contains 5 kg of saturated vapor steam at 100°C. The steam is cooled to the ambient temperature of 25°C. (a) Sketch the process with respect to the saturation lines on a T-v diagram. (b) Determine the entropy change of the steam, in kJ/K. (c) For the steam and its surroundings, determine the total entropy change or Sgen associated with this process, in kJ/K. Get 7.49 exercise solution
7–50 Steam at 6000 kPa and 500°C enters a steady-flow turbine. The steam expands in the turbine while doing work until the pressure is 1000 kPa. When the pressure is 1000 kPa, 10 percent of the steam is removed from the turbine for other uses. The remaining 90 percent of the steam continues to expand through the turbine while doing work and leaves the turbine at 10 kPa. The entire expansion process by the steam through the turbine is reversible and adiabatic. (a) Sketch the process on a T-s diagram with respect to the saturation lines. Be sure to label the data states and the lines of constant pressure. (b) If the turbine has an isentropic efficiency of 85 percent, what is the work done by the steam as it flows through the turbine per unit mass of steam flowing into the turbine, in kJ/kg? Get 7.50 exercise solution
7–51E A 1.2-ft3 well-insulated rigid can initially contains refrigerant-134a at 140 psia and 70°F. Now a crack develops in the can, and the refrigerant starts to leak out slowly, Assuming the refrigerant remaining in the can has undergone a reversible, adiabatic process, determine the final mass in the can when the pressure drops to 20 psia. Get 7.51 exercise solution
7–52C Consider two solid blocks, one hot and the other cold, brought into contact in an adiabatic container. After a while, thermal equilibrium is established in the container as a result of heat transfer. The first law requires that the amount of energy lost by the hot solid be equal to the amount of energy gained by the cold one. Does the second law require that the decrease in entropy of the hot solid be equal to the increase in entropy of the cold one? Get 7.52 exercise solution
7–53 A 50-kg copper block initially at 80°C is dropped into an insulated tank that contains 120 L of water at 25°C. Determine the final equilibrium temperature and the total entropy change for this process.

Get 7.53 exercise solution
7–54 A 25-kg iron block initially at 350°C is quenched in an insulated tank that contains 100 kg of water at 18°C. Assuming the water that vaporizes during the process condenses back in the tank, determine the total entropy change during this process. Get 7.54 exercise solution
7–55 A 20-kg aluminum block initially at 200°C is brought into contact with a 20-kg block of iron at 100°C in an insulated enclosure. Determine the final equilibrium temperature and the total entropy change for this process. Get 7.55 exercise solution
7–56 Reconsider Prob. 7–55. Using EES (or other) software, study the effect of the mass of the iron block on the final equilibrium temperature and the total entropy change for the process. Let the mass of the iron vary from 1 to 10 kg. Plot the equilibrium temperature and the total entropy change as a function of iron mass, and discuss the results. Get 7.56 exercise solution
7–57 A 50-kg iron block and a 20-kg copper block, both initially at 80°C, are dropped into a large lake at 15°C. Thermal equilibrium is established after a while as a result of heat transfer between the blocks and the lake water. Determine the total entropy change for this process. Get 7.57 exercise solution
7–58 An adiabatic pump is to be used to compress saturated liquid water at 10 kPa to a pressure to 15 MPa in a reversible manner. Determine the work input using (a) entropy data from the compressed liquid table, (b) inlet specific volume and pressure values, (c) average specific volume and pressure values. Also, determine the errors involved in parts (b) and (c).

Get 7.58 exercise solution
7–59C Prove that the two relations for entropy change of ideal gases under the constant-specific-heat assumption (Eqs. 7–33 and 7–34) are equivalent. Get 7.59 exercise solution
7–60C Starting with the second T ds relation (Eq. 7–26), obtain Eq. 7–34 for the entropy change of ideal gases under the constant-specific-heat assumption. Get 7.60 exercise solution
7–61C Some properties of ideal gases such as internal energy and enthalpy vary with temperature only [that is, u = u(T) and h = h(T)]. Is this also the case for entropy? Get 7.61 exercise solution
7–62C Starting with Eq. 7–34, obtain Eq. 7–43. Get 7.62 exercise solution
7–63C What are Pr and vr called? Is their use limited to isentropic processes? Explain. Get 7.63 exercise solution
7–64C Can the entropy of an ideal gas change during an isothermal process? Get 7.64 exercise solution
7–65C An ideal gas undergoes a process between two specified temperatures, first at constant pressure and then at constant volume. For which case will the ideal gas experience a larger entropy change? Explain. Get 7.65 exercise solution
7–66 Oxygen gas is compressed in a piston–cylinder device from an initial state of 0.8 m3/kg and 25°C to a final state of 0.1 m3/kg and 287°C. Determine the entropy change of the oxygen during this process. Assume constant specific heats. Get 7.66 exercise solution
7–67 A 1.5-m3 insulated rigid tank contains 2.7 kg of carbon dioxide at 100 kPa. Now paddle-wheel work is done on the system until the pressure in the tank rises to 150 kPa. Determine the entropy change of carbon dioxide during this process. Assume constant specific heats. Get 7.67 exercise solution
7–68 An insulated piston–cylinder device initially contains 300 L of air at 120 kPa and 17°C. Air is now heated for 15 min by a 200-W resistance heater placed inside the cylinder. The pressure of air is maintained constant during this process. Determine the entropy change of air, assuming (a) constant specific heats and (b) variable specific heats. Get 7.68 exercise solution
7–69 A piston–cylinder device contains 1.2 kg of nitrogen gas at 120 kPa and 27°C. The gas is now compressed slowly in a polytropic process during which PV1.3 = constant. The process ends when the volume is reduced by one-half. Determine the entropy change of nitrogen during this process. Get 7.69 exercise solution
7–70 Reconsider Prob. 7–69. Using EES (or other) software, investigate the effect of varying the polytropic exponent from 1 to 1.4 on the entropy change of the nitrogen. Show the processes on a common P-v diagram. Get 7.70 exercise solution
7–71E A mass of 15 lbm of helium undergoes a process from an initial state of 50 ft3/lbm and 80°F to a final state of 10 ft3/lbm and 200°F. Determine the entropy change of helium during this process, assuming (a) the process is reversible and (b) the process is irreversible. Get 7.71 exercise solution
7–72 Air is compressed in a piston–cylinder device from 90 kPa and 20°C to 400 kPa in a reversible isothermal process. Determine (a) the entropy change of air and (b) the work done. Get 7.72 exercise solution
7–73 Air is compressed steadily by a 5-kW compressor from 100 kPa and 17°C to 600 kPa and 167°C at a rate of 1.6 kg/min. During this process, some heat transfer takes place between the compressor and the surrounding medium at 17°C. Determine the rate of entropy change of air during this process.

Get 7.73 exercise solution
7–74 An insulated rigid tank is divided into two equal parts by a partition. Initially, one part contains 5 kmol of an ideal gas at 250 kPa and 40°C, and the other side is evacuated. The partition is now removed, and the gas fills the entire tank. Determine the total entropy change during this process. Get 7.74 exercise solution
7–75 Air is compressed in a piston–cylinder device from 100 kPa and 17°C to 800 kPa in a reversible, adiabatic process. Determine the final temperature and the work done during this process, assuming (a) constant specific heats and (b) variable specific heats for air. Get 7.75 exercise solution
7–76 Reconsider Prob. 7–75. Using EES (or other) software, evaluate and plot the work done and final temperature during the compression process as functions of the final pressure for the two cases as the final pressure varies from 100 to 800 kPa. Get 7.76 exercise solution
7–77 Helium gas is compressed from 90 kPa and 30°C to 450 kPa in a reversible, adiabatic process. Determine the final temperature and the work done, assuming the process takes place (a) in a piston–cylinder device and (b) in a steady-flow compressor. Get 7.77 exercise solution
7–78 An insulated rigid tank contains 4 kg of argon gas at 450 kPa and 30°C. A valve is now opened, and argon is allowed to escape until the pressure inside drops to 200 kPa. Assuming the argon remaining inside the tank has undergone a reversible, adiabatic process, determine the final mass in the tank. Get 7.78 exercise solution
7–79 Reconsider Prob. 7–78. Using EES (or other) software, investigate the effect of the final pressure on the final mass in the tank as the pressure varies from 450 to 150 kPa, and plot the results. Get 7.79 exercise solution
7–80E Air enters an adiabatic nozzle at 60 psia, 540°F, and 200 ft/s and exits at 12 psia. Assuming air to be an ideal gas with variable specific heats and disregarding any irreversibilities, determine the exit velocity of the air. Get 7.80 exercise solution
7–81 Air enters a nozzle steadily at 280 kPa and 77°C with a velocity of 50 m/s and exits at 85 kPa and 320 m/s. The heat losses from the nozzle to the surrounding medium at 20°C are estimated to be 3.2 kJ/kg. Determine (a) the exit temperature and (b) the total entropy change for this process. Get 7.81 exercise solution
7–82 Reconsider Prob. 7–81. Using EES (or other) software, study the effect of varying the surrounding medium temperature from 10 to 40°C on the exit temperature and the total entropy change for this process, and plot the results. Get 7.82 exercise solution
7–83 A container filled with 45 kg of liquid water at 95°C is placed in a 90-m3 room that is initially at 12°C. Thermal equilibrium is established after a while as a result of heat transfer between the water and the air in the room. Using constant specific heats, determine (a) the final equilibrium temperature, (b) the amount of heat transfer between the water and the air in the room, and (c) the entropy generation. Assume the room is well sealed and heavily insulated. Get 7.83 exercise solution
7–84 Air at 800 kPa and 400°C enters a steady-flow nozzle with a low velocity and leaves at 100 kPa. If the air undergoes an adiabatic expansion process through the nozzle, what is the maximum velocity of the air at the nozzle exit, in m/s? Get 7.84 exercise solution
7–85 An ideal gas at 100 kPa and 27°C enters a steady-flow compressor. The gas is compressed to 400 kPa, and 10 percent of the mass that entered the compressor is removed for some other use. The remaining 90 percent of the inlet gas is compressed to 600 kPa before leaving the compressor. The entire compression process is assumed to be reversible and adiabatic. The power supplied to the compressor is measured to be 32 kW. If the ideal gas has constant specific heats such that cv = 0.8 kJ/kg . K and cp = 1.1 kJ/kg . K, (a) sketch the compression process on a T-s diagram, (b) determine the temperature of the gas at the two compressor exits, in K, and (c) determine the mass flow rate of the gas into the compressor, in kg/s. Get 7.85 exercise solution
7–86 A constant-volume tank contains 5 kg of air at 100 kPa and 327°C. The air is cooled to the surroundings temperature of 27°C. Assume constant specific heats at 300 K. (a) Determine the entropy change of the air in the tank during the process, in kJ/K, (b) determine the net entropy change of the universe due to this process, in kJ/K, and (c) sketch the processes for the air in the tank and the surroundings on a single T-s diagram. Be sure to label the initial and final states for both processes. Get 7.86 exercise solution
7–87C In large compressors, the gas is frequently cooled while being compressed to reduce the power consumed by the compressor. Explain how cooling the gas during a compression process reduces the power consumption. Get 7.87 exercise solution
7–88C The turbines in steam power plants operate essentially under adiabatic conditions. A plant engineer suggests to end this practice. She proposes to run cooling water through the outer surface of the casing to cool the steam as it flows through the turbine. This way, she reasons, the entropy of the steam will decrease, the performance of the turbine will improve, and as a result the work output of the turbine will increase. How would you evaluate this proposal? Get 7.88 exercise solution
7–89C It is well known that the power consumed by a compressor can be reduced by cooling the gas during compression. Inspired by this, somebody proposes to cool the liquid as it flows through a pump, in order to reduce the power consumption of the pump. Would you support this proposal? Explain. Get 7.89 exercise solution
7–90 Water enters the pump of a steam power plant as saturated liquid at 20 kPa at a rate of 45 kg/s and exits at 6 MPa. Neglecting the changes in kinetic and potential energies and assuming the process to be reversible, determine the power input to the pump. Get 7.90 exercise solution
7–91 Liquid water enters a 25-kW pump at 100-kPa pressure at a rate of 5 kg/s. Determine the highest pressure the liquid water can have at the exit of the pump. Neglect the kinetic and potential energy changes of water, and take the specific volume of water to be 0.001 m3/kg. Get 7.91 exercise solution
7–92E Saturated refrigerant-134a vapor at 15 psia is compressed reversibly in an adiabatic compressor to 80 psia. Determine the work input to the compressor. What would your answer be if the refrigerant were first condensed at constant pressure before it was compressed? Get 7.92 exercise solution
7–93 Consider a steam power plant that operates between the pressure limits of 10 MPa and 20 kPa. Steam enters the pump as saturated liquid and leaves the turbine as saturated vapor. Determine the ratio of the work delivered by the turbine to the work consumed by the pump. Assume the entire cycle to be reversible and the heat losses from the pump and the turbine to be negligible. Get 7.93 exercise solution
7–94 Reconsider Prob. 7–93. Using EES (or other) software, investigate the effect of the quality of the steam at the turbine exit on the net work output. Vary the quality from 0.5 to 1.0, and plot the net work output as a function of this quality. Get 7.94 exercise solution
7–95 Liquid water at 120 kPa enters a 7-kW pump where its pressure is raised to 5 MPa. If the elevation difference between the exit and the inlet levels is 10 m, determine the highest mass flow rate of liquid water this pump can handle. Neglect the kinetic energy change of water, and take the specific volume of water to be 0.001 m3/kg. Get 7.95 exercise solution
7–96E Helium gas is compressed from 14 psia and 70°F to 120 psia at a rate of 5 ft3/s. Determine the power input to the compressor, assuming the compression process to be (a) isentropic, (b) polytropic with n = 1.2, (c) isothermal, and (d) ideal two-stage polytropic with n = 1.2. Get 7.96 exercise solution
7–97E Reconsider Prob. 7–96E. Using EES (or other) software, evaluate and plot the work of compression and entropy change of the helium as functions of the polytropic exponent as it varies from 1 to 1.667. Discuss your results. Get 7.97 exercise solution
7–98 Nitrogen gas is compressed from 80 kPa and 27°C to 480 kPa by a 10-kW compressor. Determine the mass flow rate of nitrogen through the compressor, assuming the compression process to be (a) isentropic, (b) polytropic with n = 1.3, (c) isothermal, and (d) ideal two-stage polytropic with n = 1.3. Get 7.98 exercise solution
7–99 The compression stages in the axial compressor of the industrial gas turbine are close coupled, making intercooling very impractical. To cool the air in such compressors and to reduce the compression power, it is proposed to spray water mist with drop size on the order of 5 microns into the air stream as it is compressed and to cool the air continuously as the water evaporates. Although the collision of water droplets with turbine blades is a concern, experience with steam turbines indicates that they can cope with water droplet concentrations of up to 14 percent. Assuming air is compressed isentropically at a rate of 2 kg/s from 300 K and 100 kPa to 1200 kPa and the water is injected at a temperature of 20°C at a rate of 0.2 kg/s, determine the reduction in the exit temperature of the compressed air and the compressor power saved. Assume the water vaporizes completely before leaving the compressor, and assume an average mass flow rate of 2.1 kg/s throughout the compressor. Get 7.99 exercise solution
7–100 Reconsider Prob. 7–99. The water-injected compressor is used in a gas turbine power plant. It is claimed that the power output of a gas turbine will increase because of the increase in the mass flow rate of the gas (air + water vapor) through the turbine. Do you agree? Get 7.100 exercise solution
7–101C Describe the ideal process for an (a) adiabatic turbine, (b) adiabatic compressor, and (c) adiabatic nozzle, and define the isentropic efficiency for each device. Get 7.101 exercise solution
7–102C Is the isentropic process a suitable model for compressors that are cooled intentionally? Explain. Get 7.102 exercise solution
7–103C On a T-s diagram, does the actual exit state (state 2) of an adiabatic turbine have to be on the right-hand side of the isentropic exit state (state 2s)? Why? Get 7.103 exercise solution
7–104 Steam enters an adiabatic turbine at 8 MPa and 500°C with a mass flow rate of 3 kg/s and leaves at 30 kPa. The isentropic efficiency of the turbine is 0.90. Neglecting the kinetic energy change of the steam, determine (a) the temperature at the turbine exit and (b) the power output of the turbine. Get 7.104 exercise solution
7–105 Reconsider Prob. 7–104. Using EES (or other) software, study the effect of varying the turbine isentropic efficiency from 0.75 to 1.0 on both the work done and the exit temperature of the steam, and plot your results. Get 7.105 exercise solution
7–106 Steam enters an adiabatic turbine at 7 MPa, 600°C, and 80 m/s and leaves at 50 kPa, 150°C, and 140 m/s. If the power output of the turbine is 6 MW, determine (a) the mass flow rate of the steam flowing through the turbine and (b) the isentropic efficiency of the turbine. Get 7.106 exercise solution
7–107 Argon gas enters an adiabatic turbine at 800°C and 1.5 MPa at a rate of 80 kg/min and exhausts at 200 kPa. If the power output of the turbine is 370 kW, determine the isentropic efficiency of the turbine. Get 7.107 exercise solution
7–108E Combustion gases enter an adiabatic gas turbine at 1540°F and 120 psia and leave at 60 psia with a low velocity. Treating the combustion gases as air and assuming an isentropic efficiency of 82 percent, determine the work output of the turbine. Get 7.108 exercise solution
7–109 Refrigerant-134a enters an adiabatic compressor as saturated vapor at 120 kPa at a rate of 0.3 m3/min and exits at 1-MPa pressure. If the isentropic efficiency of the compressor is 80 percent, determine (a) the temperature of the refrigerant at the exit of the compressor and (b) the power input, in kW. Also, show the process on a T-s diagram with respect to saturation lines. Get 7.109 exercise solution
7–110 Reconsider Prob. 7–109. Using EES (or other) software, redo the problem by including the effects of the kinetic energy of the flow by assuming an inletto-exit area ratio of 1.5 for the compressor when the compressor exit pipe inside diameter is 2 cm. Get 7.110 exercise solution
7–111 Air enters an adiabatic compressor at 100 kPa and 17°C at a rate of 2.4 m3/s, and it exits at 257°C. The compressor has an isentropic efficiency of 84 percent. Neglecting the changes in kinetic and potential energies, determine (a) the exit pressure of air and (b) the power required to drive the compressor. Get 7.111 exercise solution
7–112 Air is compressed by an adiabatic compressor from 95 kPa and 27°C to 600 kPa and 277°C. Assuming variable specific heats and neglecting the changes in kinetic and potential energies, determine (a) the isentropic efficiency of the compressor and (b) the exit temperature of air if the process were reversible. Answers: (a) 81.9 percent, (b) 505.5 K Get 7.112 exercise solution
7–113E Argon gas enters an adiabatic compressor at 20 psia and 90°F with a velocity of 60 ft/s, and it exits at 200 psia and 240 ft/s. If the isentropic efficiency of the compressor is 80 percent, determine (a) the exit temperature of the argon and (b) the work input to the compressor. Get 7.113 exercise solution
7–114 Carbon dioxide enters an adiabatic compressor at 100 kPa and 300 K at a rate of 1.8 kg/s and exits at 600 kPa and 450 K. Neglecting the kinetic energy changes, determine the isentropic efficiency of the compressor. Get 7.114 exercise solution
7–115E Air enters an adiabatic nozzle at 60 psia and 1020°F with low velocity and exits at 800 ft/s. If the isentropic efficiency of the nozzle is 90 percent, determine the exit temperature and pressure of the air. Get 7.115 exercise solution
7–116E Reconsider Prob. 7–115E. Using EES (or other) software, study the effect of varying the nozzle isentropic efficiency from 0.8 to 1.0 on both the exit temperature and pressure of the air, and plot the results. Get 7.116 exercise solution
7–117 Hot combustion gases enter the nozzle of a turbojet engine at 260 kPa, 747°C, and 80 m/s, and they exit at a pressure of 85 kPa. Assuming an isentropic efficiency of 92 percent and treating the combustion gases as air, determine (a) the exit velocity and (b) the exit temperature. Get 7.117 exercise solution
7–118 Refrigerant-134a is throttled from 900 kPa and 35°C to 200 kPa. Heat is lost from the refrigerant in the amount of 0.8 kJ/kg to the surroundings at 25°C. Determine (a) the exit temperature of the refrigerant and (b) the entropy generation during this process. Get 7.118 exercise solution
7–119 Steam enters an adiabatic turbine steadily at 7 MPa, 500°C, and 45 m/s, and leaves at 100 kPa and 75 m/s. If the power output of the turbine is 5 MW and the isentropic efficiency is 77 percent, determine (a) the mass flow rate of steam through the turbine, (b) the temperature at the turbine exit, and (c) the rate of entropy generation during this process Get 7.119 exercise solution
7–120 Air enters a compressor steadily at the ambient conditions of 100 kPa and 22°C and leaves at 800 kPa. Heat is lost from the compressor in the amount of 120 kJ/kg and the air experiences an entropy decrease of 0.40 kJ/kg . K. Using constant specific heats, determine (a) the exit temperature of the air, (b) the work input to the compressor, and (c) the entropy generation during this process. Get 7.120 exercise solution
7–121 A rigid tank contains 7.5 kg of saturated water mixture at 400 kPa. A valve at the bottom of the tank is now opened, and liquid is withdrawn from the tank. Heat is transferred to the steam such that the pressure inside the tank remains constant. The valve is closed when no liquid is left in the tank. If it is estimated that a total of 5 kJ of heat is transferred to the tank, determine (a) the quality of steam in the tank at the initial state, (b) the amount of mass that has escaped, and (c) the entropy generation during this process if heat is supplied to the tank from a source at 500°C. Get 7.121 exercise solution
7–122 Consider a family of four, with each person taking a 5-min shower every morning. The average flow rate through the shower head is 12 L/min. City water at 15°C is heated to 55°C in an electric water heater and tempered to 42°C by cold water at the T-elbow of the shower before being routed to the shower head. Determine the amount of entropy generated by this family per year as a result of taking daily showers. Get 7.122 exercise solution
7–123 Steam is to be condensed in the condenser of a steam power plant at a temperature of 60°C with cooling water from a nearby lake, which enters the tubes of the condenser at 18°C at a rate of 75 kg/s and leaves at 27°C. Assuming the condenser to be perfectly insulated, determine (a) the rate of condensation of the steam and (b) the rate of entropy generation in the condenser. Get 7.123 exercise solution
7–124 A well-insulated heat exchanger is to heat water (cp = 4.18 kJ/kg · °C) from 25 to 60°C at a rate of 0.50 kg/s. The heating is to be accomplished by geothermal water (cp = 4.31 kJ/kg · °C) available at 140°C at a mass flow rate of 0.75 kg/s. Determine (a) the rate of heat transfer and (b) the rate of entropy generation in the heat exchanger. Get 7.124 exercise solution
7–125 An adiabatic heat exchanger is to cool ethylene glycol (cp = 2.56 kJ/kg · °C) flowing at a rate of 2 kg/s from 80 to 40°C by water (cp = 4.18 kJ/kg · °C) that enters at 20°C and leaves at 55°C. Determine (a) the rate of heat transfer and (b) the rate of entropy generation in the heat exchanger. Get 7.125 exercise solution
7–126 A well-insulated, thin-walled, double-pipe, counterflow heat exchanger is to be used to cool oil (cp = 2.20 kJ/kg · °C) from 150°C to 40°C at a rate of 2 kg/s by water (cp = 4.18 kJ/kg · °C) that enters at 22°C at a rate of 1.5 kg/s. Determine (a) the rate of heat transfer and (b) the rate of entropy generation in the heat exchanger. Get 7.126 exercise solution
7–127 Cold water (cp = 4.18 kJ/kg · °C) leading to a shower enters a well-insulated, thin-walled, double-pipe, counter-flow heat exchanger at 15°C at a rate of 0.25 kg/s and is heated to 45°C by hot water (cp = 4.19 kJ/kg · °C) that enters at 100°C at a rate of 3 kg/s. Determine (a) the rate of heat transfer and (b) the rate of entropy generation in the heat exchanger.

Get 7.127 exercise solution
7–128 Air (cp = 1.005 kJ/kg · °C) is to be preheated by hot exhaust gases in a cross-flow heat exchanger before it enters the furnace. Air enters the heat exchanger at 95 kPa and 20°C at a rate of 1.6 m3/s. The combustion gases (cp = 1.10 kJ/kg · °C) enter at 180°C at a rate of 2.2 kg/s and leave at 95°C. Determine (a) the rate of heat transfer to the air, (b) the outlet temperature of the air, and (c) the rate of entropy generation. Get 7.128 exercise solution
7–129 A well-insulated, shell-and-tube heat exchanger is used to heat water (cp = 4.18 kJ/kg · °C) in the tubes from 20 to 70°C at a rate of 4.5 kg/s. Heat is supplied by hot oil (cp = 2.30 kJ/kg · °C) that enters the shell side at 170°C at a rate of 10 kg/s. Disregarding any heat loss from the heat exchanger, determine (a) the exit temperature of the oil and (b) the rate of entropy generation in the heat exchanger. Get 7.129 exercise solution
7–130E Steam is to be condensed on the shell side of a heat exchanger at 120°F. Cooling water enters the tubes at 60°F at a rate of 92 lbm/s and leaves at 73°F. Assuming the heat exchanger to be well-insulated, determine (a) the rate of heat transfer in the heat exchanger and (b) the rate of entropy generation in the heat exchanger. Get 7.130 exercise solution
7–131 Chickens with an average mass of 2.2 kg and average specific heat of 3.54 kJ/kg · °C are to be cooled by chilled water that enters a continuous-flow-type immersion chiller at 0.5°C and leaves at 2.5°C. Chickens are dropped into the chiller at a uniform temperature of 15°C at a rate of 250 chickens per hour and are cooled to an average temperature of 3°C before they are taken out. The chiller gains heat from the surroundings at 25°C at a rate of 150 kJ/h. Determine (a) the rate of heat removal from the chickens, in kW, and (b) the rate of entropy generation during this chilling process. Get 7.131 exercise solution
7–132 In a dairy plant, milk at 4°C is pasteurized continuously at 72°C at a rate of 12 L/s for 24 hours a day and 365 days a year. The milk is heated to the pasteurizing temperature by hot water heated in a natural-gas-fired boiler that has an efficiency of 82 percent. The pasteurized milk is then cooled by cold water at 18°C before it is finally refrigerated back to 4°C. To save energy and money, the plant installs a regenerator that has an effectiveness of 82 percent. If the cost of natural gas is $1.04/therm (1 therm = 105,500 kJ), determine how much energy and money the regenerator will save this company per year and the annual reduction in entropy generation.
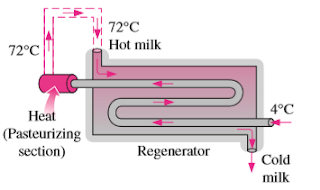
Get 7.132 exercise solution
7–133 Stainless-steel ball bearings (r = 8085 kg/m3 and cp = 0.480 kJ/kg · °C) having a diameter of 1.2 cm are to be quenched in water at a rate of 1400 per minute. The balls leave the oven at a uniform temperature of 900°C and are exposed to air at 30°C for a while before they are dropped into the water. If the temperature of the balls drops to 850°C prior to quenching, determine (a) the rate of heat transfer from the balls to the air and (b) the rate of entropy generation due to heat loss from the balls to the air. Get 7.133 exercise solution
7–134 Carbon-steel balls (r = 7833 kg/m3 and cp = 0.465 kJ/kg · °C) 8 mm in diameter are annealed by heating them first to 900°C in a furnace and then allowing them to cool slowly to 100°C in ambient air at 35°C. If 2500 balls are to be annealed per hour, determine (a) the rate of heat transfer from the balls to the air and (b) the rate of entropy generation due to heat loss from the balls to the air. Get 7.134 exercise solution
7–135 An ordinary egg can be approximated as a 5.5-cmdiameter sphere. The egg is initially at a uniform temperature of 8°C and is dropped into boiling water at 97°C. Taking the properties of the egg to be r = 1020 kg/m3 and cp = 3.32 kJ/kg · °C, determine (a) how much heat is transferred to the egg by the time the average temperature of the egg rises to 70°C and (b) the amount of entropy generation associated with this heat transfer process. Get 7.135 exercise solution
7–136E In a production facility, 1.2-in.-thick, 2-ft x 2-ft square brass plates (r = 532.5 lbm/ft3 and cp = 0.091 Btu/lbm · °F) that are initially at a uniform temperature of 75°F are heated by passing them through an oven at 1300°F at a rate of 450 per minute. If the plates remain in the oven until their average temperature rises to 1000°F, determine (a) the rate of heat transfer to the plates in the furnace and (b) the rate of entropy generation associated with this heat transfer process. Get 7.136 exercise solution
7–137 Long cylindrical steel rods (r = 7833 kg/m3 and cp = 0.465 kJ/kg · °C) of 10-cm diameter are heat treated by drawing them at a velocity of 3 m/min through a 7-m-long oven maintained at 900°C. If the rods enter the oven at 30°C and leave at 700°C, determine (a) the rate of heat transfer to the rods in the oven and (b) the rate of entropy generation associated with this heat transfer process. Get 7.137 exercise solution
7–138 The inner and outer surfaces of a 5-m x 7-m brick wall of thickness 20 cm are maintained at temperatures of 20°C and 5°C, respectively. If the rate of heat transfer through the wall is 1515 W, determine the rate of entropy generation within the wall. Get 7.138 exercise solution
7–139 For heat transfer purposes, a standing man can be modeled as a 30-cm-diameter, 170-cm-long vertical cylinder with both the top and bottom surfaces insulated and with the side surface at an average temperature of 34°C. If the rate of heat loss from this man to the environment at 20°C is 336 W, determine the rate of entropy transfer from the body of this person accompanying heat transfer, in W/K. Get 7.139 exercise solution
7–140 A 1000-W iron is left on the ironing board with its base exposed to the air at 20°C. If the surface temperature is 400°C, determine the rate of entropy generation during this process in steady operation. How much of this entropy generation occurs within the iron? Get 7.140 exercise solution
7–141E A frictionless piston–cylinder device contains saturated liquid water at 25-psia pressure. Now 400 Btu of heat is transferred to water from a source at 900°F, and part of the liquid vaporizes at constant pressure. Determine the total entropy generated during this process, in Btu/R. Get 7.141 exercise solution
7–142E Steam enters a diffuser at 20 psia and 240°F with a velocity of 900 ft/s and exits as saturated vapor at 240°F and 100 ft/s. The exit area of the diffuser is 1 ft2. Determine (a) the mass flow rate of the steam and (b) the rate of entropy generation during this process. Assume an ambient temperature of 77°F. Get 7.142 exercise solution
7–143 Steam expands in a turbine steadily at a rate of 25,000 kg/h, entering at 6 MPa and 450°C and leaving at 20 kPa as saturated vapor. If the power generated by the turbine is 4 MW, determine the rate of entropy generation for this process. Assume the surrounding medium is at 25°C. Get 7.143 exercise solution
7–144 A hot-water stream at 70°C enters an adiabatic mixing chamber with a mass flow rate of 3.6 kg/s, where it is mixed with a stream of cold water at 20°C. If the mixture leaves the chamber at 42°C, determine (a) the mass flow rate of the cold water and (b) the rate of entropy generation during this adiabatic mixing process. Assume all the streams are at a pressure of 200 kPa. Get 7.144 exercise solution
7–145 Liquid water at 200 kPa and 20°C is heated in a chamber by mixing it with superheated steam at 200 kPa and 150°C. Liquid water enters the mixing chamber at a rate of 2.5 kg/s, and the chamber is estimated to lose heat to the surrounding air at 25°C at a rate of 1200 kJ/min. If the mixture leaves the mixing chamber at 200 kPa and 60°C, determine (a) the mass flow rate of the superheated steam and (b) the rate of entropy generation during this mixing process. Get 7.145 exercise solution
7–146 A 0.3-m3 rigid tank is filled with saturated liquid water at 150°C. A valve at the bottom of the tank is now opened, and one-half of the total mass is withdrawn from the tank in the liquid form. Heat is transferred to water from a source at 200°C so that the temperature in the tank remains constant. Determine (a) the amount of heat transfer and (b) the total entropy generation for this process. Get 7.146 exercise solution
7–147E An iron block of unknown mass at 185°F is dropped into an insulated tank that contains 0.8 ft3 of water at 70°F. At the same time, a paddle wheel driven by a 200-W motor is activated to stir the water. Thermal equilibrium is established after 10 min with a final temperature of 75°F. Determine (a) the mass of the iron block and (b) the entropy generated during this process. Get 7.147 exercise solution
7–148E Air enters a compressor at ambient conditions of 15 psia and 60°F with a low velocity and exits at 150 psia, 620°F, and 350 ft/s. The compressor is cooled by the ambient air at 60°F at a rate of 1500 Btu/min. The power input to the compressor is 400 hp. Determine (a) the mass flow rate of air and (b) the rate of entropy generation. Get 7.148 exercise solution
7–149 Steam enters an adiabatic nozzle at 4 MPa and 450°C with a velocity of 70 m/s and exits at 3 MPa and 320 m/s. If the nozzle has an inlet area of 7 cm2, determine (a) the exit temperature and (b) the rate of entropy generation for this process. Get 7.149 exercise solution
7–150 Compressed air is one of the key utilities in manufacturing facilities, and the total installed power of compressedair systems in the United States is estimated to be about 20 million horsepower. Assuming the compressors to operate at full load during one-third of the time on average and the average motor efficiency to be 85 percent, determine how much energy and money will be saved per year if the energy consumed by compressors is reduced by 5 percent as a result of implementing some conservation measures. Take the unit cost of electricity to be $0.07/kWh. Get 7.150 exercise solution
7–151 The energy used to compress air in the United States is estimated to exceed one-half quadrillion (0.5 1015) kJ per year. It is also estimated that 10 to 40 percent of the compressed air is lost through leaks. Assuming, on average, 20 percent of the compressed air is lost through air leaks and the unit cost of electricity is $0.07/kWh, determine the amount and cost of electricity wasted per year due to air leaks. Get 7.151 exercise solution
7–152 The compressed-air requirements of a plant at sea level are being met by a 125-hp compressor that takes in air at the local atmospheric pressure of 101.3 kPa and the average temperature of 15°C and compresses it to 900 kPa. An investigation of the compressed-air system and the equipment using the compressed air reveals that compressing the air to 750 kPa is sufficient for this plant. The compressor operates 3500 h/yr at 75 percent of the rated load and is driven by an electric motor that has an efficiency of 88 percent. Taking the price of electricity to be $0.085/kWh, determine the amount of energy and money saved as a result of reducing the pressure of the compressed air. Get 7.152 exercise solution
7–153 A 150-hp compressor in an industrial facility is housed inside the production area where the average temperature during operating hours is 25°C. The average temperature outdoors during the same hours is 10°C. The compressor operates 4500 h/yr at 85 percent of rated load and is driven by an electric motor that has an efficiency of 90 percent. Taking the price of electricity to be $0.07/kWh, determine the amount of energy and money saved as a result of drawing outside air to the compressor instead of using the inside air. Get 7.153 exercise solution
7–154 The compressed-air requirements of a plant are being met by a 100-hp screw compressor that runs at full load during 40 percent of the time and idles the rest of the time during operating hours. The compressor consumes 35 percent of the rated power when idling and 90 percent of the power when compressing air. The annual operating hours of the facility are 3800 h, and the unit cost of electricity is $0.075/kWh. It is determined that the compressed-air requirements of the facility during 60 percent of the time can be met by a 25hp reciprocating compressor that consumes 95 percent of the rated power when compressing air and no power when not compressing air. It is estimated that the 25-hp compressor runs 85 percent of the time. The efficiencies of the motors of the large and the small compressors at or near full load are 0.90 and 0.88, respectively. The efficiency of the large motor at 35 percent load is 0.82. Determine the amount of energy and money saved as a result of switching to the 25-hp compressor during 60 percent of the time. Get 7.154 exercise solution
7–155 The compressed-air requirements of a plant are being met by a 125-hp screw compressor. The facility stops production for one hour every day, including weekends, for lunch break, but the compressor is kept operating. The compressor consumes 35 percent of the rated power when idling, and the unit cost of electricity is $0.09/kWh. Determine the amount of energy and money saved per year as a result of turning the compressor off during lunch break. Take the efficiency of the motor at part load to be 84 percent. Get 7.155 exercise solution
7–156 The compressed-air requirements of a plant are met by a 150-hp compressor equipped with an intercooler, an aftercooler, and a refrigerated dryer. The plant operates 4800 h/yr, but the compressor is estimated to be compressing air during only one-third of the operating hours, that is, 1600 hours a year. The compressor is either idling or is shut off the rest of the time. Temperature measurements and calculations indicate that 40 percent of the energy input to the compressor is removed from the compressed air as heat in the aftercooler. The COP of the refrigeration unit is 3.5, and the cost of electricity is $0.06/kWh. Determine the amount of the energy and money saved per year as a result of cooling the compressed air before it enters the refrigerated dryer. Get 7.156 exercise solution
7–157 The 1800-rpm, 150-hp motor of a compressor is burned out and is to be replaced by either a standard motor that has a full-load efficiency of 93.0 percent and costs $9031 or a high-efficiency motor that has an efficiency of 96.2 percent and costs $10,942. The compressor operates 4368 h/yr at full load, and its operation at part load is negligible. If the cost of electricity is $0.075/kWh, determine the amount of energy and money this facility will save by purchasing the highefficiency motor instead of the standard motor. Also, determine if the savings from the high-efficiency motor justify the price differential if the expected life of the motor is 10 years. Ignore any possible rebates from the local power company. Get 7.157 exercise solution
7–158 The space heating of a facility is accomplished by natural gas heaters that are 80 percent efficient. The compressed air needs of the facility are met by a large liquid-cooled compressor. The coolant of the compressor is cooled by air in a liquid-to-air heat exchanger whose airflow section is 1.0-m high and 1.0-m wide. During typical operation, the air is heated from 20 to 52°C as it flows through the heat exchanger. The average velocity of air on the inlet side is measured to be 3 m/s. The compressor operates 20 hours a day and 5 days a week throughout the year. Taking the heating season to be 6 months (26 weeks) and the cost of the natural gas to be $1.00/therm (1 therm = 100,000 Btu = 105,500 kJ), determine how much money will be saved by diverting the compressor waste heat into the facility during the heating season. Get 7.158 exercise solution
7–159 The compressors of a production facility maintain the compressed-air lines at a (gage) pressure of 850 kPa at 1400m elevation, where the atmospheric pressure is 85.6 kPa. The average temperature of air is 15°C at the compressor inlet and 25°C in the compressed-air lines. The facility operates 4200 h/yr, and the average price of electricity is $0.07/kWh. Taking the compressor efficiency to be 0.8, the motor efficiency to be 0.93, and the discharge coefficient to be 0.65, determine the energy and money saved per year by sealing a leak equivalent to a 5-mm-diameter hole on the compressed-air line. Get 7.159 exercise solution
7–160 A piston–cylinder device contains steam that undergoes a reversible thermodynamic cycle. Initially the steam is at 400 kPa and 350°C with a volume of 0.3 m3. The steam is first expanded isothermally to 150 kPa, then compressed adiabatically to the initial pressure, and finally compressed at the constant pressure to the initial state. Determine the net work and heat transfer for the cycle after you calculate the work and heat interaction for each process. Get 7.160 exercise solution
7–161 Determine the work input and entropy generation during the compression of steam from 100 kPa to 1 MPa in (a) an adiabatic pump and (b) an adiabatic compressor if the inlet state is saturated liquid in the pump and saturated vapor in the compressor and the isentropic efficiency is 85 percent for both devices. Get 7.161 exercise solution
7–162 A rigid tank contains 1.5 kg of water at 120°C and 500 kPa. Now 22 kJ of shaft work is done on the system and the final temperature in the tank is 95°C. If the entropy change of water is zero and the surroundings are at 15°C, determine (a) the final pressure in the tank, (b) the amount of heat transfer between the tank and the surroundings, and (c) the entropy generation during this process. Get 7.162 exercise solution
7–163 A horizontal cylinder is separated into two compartments by an adiabatic, frictionless piston. One side contains 0.2 m3 of nitrogen and the other side contains 0.1 kg of helium, both initially at 20°C and 95 kPa. The sides of the cylinder and the helium end are insulated. Now heat is added to the nitrogen side from a reservoir at 500°C until the pressure of the helium rises to 120 kPa. Determine (a) the final temperature of the helium, (b) the final volume of the nitrogen, (c) the heat transferred to the nitrogen, and (d) the entropy generation during this process. Get 7.163 exercise solution
7–164 A 0.8-m3 rigid tank contains carbon dioxide (CO2) gas at 250 K and 100 kPa. A 500-W electric resistance heater placed in the tank is now turned on and kept on for 40 min after which the pressure of CO2 is measured to be 175 kPa. Assuming the surroundings to be at 300 K and using constant specific heats, determine (a) the final temperature of CO2, (b) the net amount of heat transfer from the tank, and (c) the entropy generation during this process. Get 7.164 exercise solution
7–165 Helium gas is throttled steadily from 500 kPa and 70°C. Heat is lost from the helium in the amount of 2.5 kJ/kg to the surroundings at 25°C and 100 kPa. If the entropy of the helium increases by 0.25 kJ/kg . K in the valve, determine (a) the exit pressure and temperature and (b) the entropy generation during this process. Get 7.165 exercise solution
7–166 Refrigerant-134a enters a compressor as a saturated vapor at 200 kPa at a rate of 0.03 m3/s and leaves at 700 kPa. The power input to the compressor is 10 kW. If the surroundings at 20°C experience an entropy increase of 0.008 kW/K, determine (a) the rate of heat loss from the compressor, (b) the exit temperature of the refrigerant, and (c) the rate of entropy generation. Get 7.166 exercise solution
7–167 Air at 500 kPa and 400 K enters an adiabatic nozzle at a velocity of 30 m/s and leaves at 300 kPa and 350 K. Using variable specific heats, determine (a) the isentropic efficiency, (b) the exit velocity, and (c) the entropy generation. Get 7.167 exercise solution
7–168 Show that the difference between the reversible steady-flow work and reversible moving boundary work is equal to the flow energy. Get 7.168 exercise solution
7–169 An insulated tank containing 0.4 m3 of saturated water vapor at 500 kPa is connected to an initially evacuated, insulated piston–cylinder device. The mass of the piston is such that a pressure of 150 kPa is required to raise it. Now the valve is opened slightly, and part of the steam flows to the cylinder, raising the piston. This process continues until the pressure in the tank drops to 150 kPa. Assuming the steam that remains in the tank to have undergone a reversible adiabatic process, determine the final temperature (a) in the rigid tank and (b) in the cylinder. Get 7.169 exercise solution
7–170 One ton of liquid water at 80°C is brought into a well-insulated and well-sealed 4-m x 5-m x 7-m room initially at 22°C and 100 kPa. Assuming constant specific heats for both air and water at room temperature, determine (a) the final equilibrium temperature in the room and (b) the total entropy change during this process, in kJ/K. Get 7.170 exercise solution
7–171E A piston–cylinder device initially contains 15 ft3 of helium gas at 25 psia and 70°F. Helium is now compressed in a polytropic process (PVn = constant) to 70 psia and 300°F. Determine (a) the entropy change of helium, (b) the entropy change of the surroundings, and (c) whether this process is reversible, irreversible, or impossible. Assume the surroundings are at 70°F. Get 7.171 exercise solution
7-172 Air is compressed steadily by a compressor from 100 kPa and 17°C to 700 kPa at a rate of 5 kg/min. Determine the minimum power input required if the process is (a) adiabatic and (b) isothermal. Assume air to be an ideal gas with variable specific heats, and neglect the changes in kinetic and potential energies. Get 7.172 exercise solution
7–173 Air enters a two-stage compressor at 100 kPa and 27°C and is compressed to 900 kPa. The pressure ratio across each stage is the same, and the air is cooled to the initial temperature between the two stages. Assuming the compression process to be isentropic, determine the power input to the compressor for a mass flow rate of 0.02 kg/s. What would your answer be if only one stage of compression were used? Get 7.173 exercise solution
7–174 Consider a three-stage isentropic compressor with two intercoolers that cool the gas to the initial temperature between the stages. Determine the two intermediate pressures (Px and Py) in terms of inlet and exit pressures (P1 and P2) that will minimize the work input to the compressor Get 7.174 exercise solution
7–175 Steam at 6 MPa and 500°C enters a two-stage adiabatic turbine at a rate of 15 kg/s. Ten percent of the steam is extracted at the end of the first stage at a pressure of 1.2 MPa for other use. The remainder of the steam is further expanded in the second stage and leaves the turbine at 20 kPa. Determine the power output of the turbine, assuming (a) the process is reversible and (b) the turbine has an isentropic efficiency of 88 percent. Get 7.175 exercise solution
7–176 Steam enters a two-stage adiabatic turbine at 8 MPa and 550°C. It expands in the first stage to a pressure of 2 MPa. Then steam is reheated at constant pressure to 550°C before it is expanded in a second stage to a pressure of 200 kPa. The power output of the turbine is 80 MW. Assuming an isentropic efficiency of 84 percent for each stage of the turbine, determine the required mass flow rate of steam. Also, show the process on a T-s diagram with respect to saturation lines. Answer: 85.8 kg/s Get 7.176 exercise solution
7–177 Refrigerant-134a at 140 kPa and -10°C is compressed by an adiabatic 0.7-kW compressor to an exit state of 700 kPa and 50°C. Neglecting the changes in kinetic and potential energies, determine (a) the isentropic efficiency of the compressor, (b) the volume flow rate of the refrigerant at the compressor inlet, in L/min, and (c) the maximum volume flow rate at the inlet conditions that this adiabatic 0.7-kW compressor can handle without violating the second law. Get 7.177 exercise solution
7–178E Helium gas enters a nozzle whose isentropic efficiency is 94 percent with a low velocity, and it exits at 14 psia, 180°F, and 1000 ft/s. Determine the pressure and temperature at the nozzle inlet. Get 7.178 exercise solution
7–179 An adiabatic air compressor is to be powered by a direct-coupled adiabatic steam turbine that is also driving a generator. Steam enters the turbine at 12.5 MPa and 500°C at a rate of 25 kg/s and exits at 10 kPa and a quality of 0.92. Air enters the compressor at 98 kPa and 295 K at a rate of 10 kg/s and exits at 1 MPa and 620 K. Determine (a) the net power delivered to the generator by the turbine and (b) the rate of entropy generation within the turbine and the compressor during this process. Get 7.179 exercise solution
7–180 Reconsider Prob. 7–179. Using EES (or other) software, determine the isentropic efficiencies for the compressor and turbine. Then use EES to study how varying the compressor efficiency over the range 0.6 to 0.8 and the turbine efficiency over the range 0.7 to 0.95 affect the net work for the cycle and the entropy generated for the process. Plot the net work as a function of the compressor efficiency for turbine efficiencies of 0.7, 0.8, and 0.9, and discuss your results. Get 7.180 exercise solution
7–181 Consider two bodies of identical mass m and specific heat c used as thermal reservoirs (source and sink) for a heat engine. The first body is initially at an absolute temperature T1 while the second one is at a lower absolute temperature T2. Heat is transferred from the first body to the heat engine, which rejects the waste heat to the second body. The process continues until the final temperatures of the two bodies Tf become equal. Show that when the heat engine produces the maximum possible work. Get 7.181 exercise solution
7–182 The explosion of a hot water tank in a school in Spencer, Oklahoma, in 1982 killed 7 people while injuring 33 others. Although the number of such explosions has decreased dramatically since the development of the ASME Pressure Vessel Code, which requires the tanks to be designed to withstand four times the normal operating pressures, they still occur as a result of the failure of the pressure relief valves and thermostats. When a tank filled with a highpressure and high-temperature liquid ruptures, the sudden drop of the pressure of the liquid to the atmospheric level causes part of the liquid to flash into vapor, and thus to experience a huge rise in its volume. The resulting pressure wave that propagates rapidly can cause considerable damage. Considering that the pressurized liquid in the tank eventually reaches equilibrium with its surroundings shortly after the explosion, the work that a pressurized liquid would do if allowed to expand reversibly and adiabatically to the pressure of the surroundings can be viewed as the explosive energy of the pressurized liquid. Because of the very short time period of the explosion and the apparent calm afterward, the explosion process can be considered to be adiabatic with no changes in kinetic and potential energies and no mixing with the air. Consider a 80-L hot-water tank that has a working pressure of 0.5 MPa. As a result of some malfunction, the pressure in the tank rises to 2 MPa, at which point the tank explodes. Taking the atmospheric pressure to be 100 kPa and assuming the liquid in the tank to be saturated at the time of explosion, determine the total explosion energy of the tank in terms of the TNT equivalence. (The explosion energy of TNT is about 3250 kJ/kg, and 5 kg of TNT can cause total destruction of unreinforced structures within about a 7-m radius.) Get 7.182 exercise solution
7–183 Using the arguments in the Prob. 7–182, determine the total explosion energy of a 0.35-L canned drink that explodes at a pressure of 1.2 MPa. To how many kg of TNT is this explosion energy equivalent? Get 7.183 exercise solution
7–184 Demonstrate the validity of the Clausius inequality using a reversible and an irreversible heat engine operating between the same two thermal energy reservoirs at constant temperatures of TL and TH. Get 7.184 exercise solution
7–185 The inner and outer surfaces of a 2-m x 2-m window glass in winter are 10°C and 3°C, respectively. If the rate of heat loss through the window is 3.2 kJ/s, determine the amount of heat loss, in kilojoules, through the glass over a period of 5 h. Also, determine the rate of entropy generation during this process within the glass. Get 7.185 exercise solution
7–186 Two rigid tanks are connected by a valve. Tank A is insulated and contains 0.2 m3 of steam at 400 kPa and 80 percent quality. Tank B is uninsulated and contains 3 kg of steam at 200 kPa and 250°C. The valve is now opened, and steam flows from tank A to tank B until the pressure in tank A drops to 300 kPa. During this process 600 kJ of heat is transferred from tank B to the surroundings at 0°C. Assuming the steam remaining inside tank A to have undergone a reversible adiabatic process, determine (a) the final temperature in each tank and (b) the entropy generated during this process. Get 7.186 exercise solution
7–187 Heat is transferred steadily to boiling water in the pan through its flat bottom at a rate of 500 W. If the temperatures of the inner and outer surfaces of the bottom of the tank are 104°C and 105°C, respectively, determine the rate of entropy generation within bottom of the pan, in W/K. Get 7.187 exercise solution
7–188 A 1200-W electric resistance heating element whose diameter is 0.5 cm is immersed in 40 kg of water initially at 20°C. Assuming the water container is well-insulated,determine how long it will take for this heater to raise the water temperature to 50°C. Also, determine the entropy generated during this process, in kJ/K. Get 7.188 exercise solution
7–189 A hot-water pipe at 80°C is losing heat to the surrounding air at 5°C at a rate of 2200 W. Determine the rate of entropy generation in the surrounding air, in W/K. Get 7.189 exercise solution
7–190 In large steam power plants, the feedwater is frequently heated in closed feedwater heaters, which are basically heat exchangers, by steam extracted from the turbine at some stage. Steam enters the feedwater heater at 1 MPa and 200°C and leaves as saturated liquid at the same pressure. Feedwater enters the heater at 2.5 MPa and 50°C and leaves 10°C below the exit temperature of the steam. Neglecting any heat losses from the outer surfaces of the heater, determine (a) the ratio of the mass flow rates of the extracted steam and the feedwater heater and (b) the total entropy change for this process per unit mass of the feedwater. Get 7.190 exercise solution
7–191 Reconsider Prob. 7–190. Using EES (or other) software, investigate the effect of the state of the steam at the inlet of the feedwater heater. Assume the entropy of the extraction steam is constant at the value for 1 MPa, 200°C and decrease the extraction steam pressure from 1 MPa to 100 kPa. Plot both the ratio of the mass flow rates of the extracted steam and the feedwater heater and the total entropy change for this process per unit mass of the feedwater as functions of the extraction pressure. Get 7.191 exercise solution
7–192E A 3-ft3 rigid tank initially contains refrigerant-134a at 100 psia and 100 percent quality. The tank is connected by a valve to a supply line that carries refrigerant-134a at 140 psia and 80°F. The valve is now opened, allowing the refrigerant to enter the tank, and is closed when it is observed that the tank contains only saturated liquid at 120 psia. Determine (a) the mass of the refrigerant that entered the tank, (b) the amount of heat transfer with the surroundings at 110°F, and (c) the entropy generated during this process. Get 7.192 exercise solution
7–193 During a heat transfer process, the entropy change of incompressible substances, such as liquid water, can be determined from DS = mcavg ln(T2/T1). Show that for thermal energy reservoirs, such as large lakes, this relation reduces to DS = Q/T. Get 7.193 exercise solution
7–194 The inner and outer glasses of a 2-m x 2-m doublepane window are at 18°C and 6°C, respectively. If the glasses are very nearly isothermal and the rate of heat transfer through the window is 110 W, determine the rates of entropy transfer through both sides of the window and the rate of entropy generation within the window, in W/K. Get 7.194 exercise solution
7–195 A well-insulated 4-m x 4-m x 5-m room initially at 10°C is heated by the radiator of a steam heating system. The radiator has a volume of 15 L and is filled with superheated vapor at 200 kPa and 200°C. At this moment both the inlet and the exit valves to the radiator are closed. A 120-W fan is used to distribute the air in the room. The pressure of the steam is observed to drop to 100 kPa after 30 min as a result of heat transfer to the room. Assuming constant specific heats for air at room temperature, determine (a) the average temperature of air in 30 min, (b) the entropy change of the steam, (c) the entropy change of the air in the room, and (d) the entropy generated during this process, in kJ/K. Assume the air pressure in the room remains constant at 100 kPa at all times. Get 7.195 exercise solution
7–196 A passive solar house that is losing heat to the outdoors at 3°C at an average rate of 50,000 kJ/h is maintained at 22°C at all times during a winter night for 10 h. The house is to be heated by 50 glass containers, each containing 20 L of water that is heated to 80°C during the day by absorbing solar energy. A thermostat controlled 15 kW backup electric resistance heater turns on whenever necessary to keep the house at 22°C. Determine how long the electric heating system was on that night and the amount of entropy generated during the night. Get 7.196 exercise solution
7–197E A 15-ft3 steel container that has a mass of 75 lbm when empty is filled with liquid water. Initially, both the steel tank and the water are at 120°F. Now heat is transferred, and the entire system cools to the surrounding air temperature of 70°F. Determine the total entropy generated during this process. Get 7.197 exercise solution
7–198 Air enters the evaporator section of a window air conditioner at 100 kPa and 27°C with a volume flow rate of 6 m3/min. The refrigerant-134a at 120 kPa with a quality of 0.3 enters the evaporator at a rate of 2 kg/min and leaves as saturated vapor at the same pressure. Determine the exit temperature of the air and the rate of entropy generation for this process, assuming (a) the outer surfaces of the air conditioner are insulated and (b) heat is transferred to the evaporator of the air conditioner from the surrounding medium at 32°C at a rate of 30 kJ/min. Get 7.198 exercise solution
7–199 A 4-m x 5-m x 7-m well-sealed room is to be heated by 1500 kg of liquid water contained in a tank that is placed in the room. The room is losing heat to the outside air at 5°C at an average rate of 10,000 kJ/h. The room is initially at 20°C and 100 kPa and is maintained at a temperature of 20°C at all times. If the hot water is to meet the heating requirements of this room for a 24-h period, determine (a) the minimum temperature of the water when it is first brought into the room and (b) the entropy generated during a 24-h period. Assume constant specific heats for both air and water at room temperature. Get 7.199 exercise solution
7–200 Consider a well-insulated horizontal rigid cylinder that is divided into two compartments by a piston that is free to move but does not allow either gas to leak into the other side. Initially, one side of the piston contains 1 m3 of N2 gas at 500 kPa and 80°C while the other side contains 1 m3 of He gas at 500 kPa and 25°C. Now thermal equilibrium is established in the cylinder as a result of heat transfer through the piston. Using constant specific heats at room temperature, determine (a) the final equilibrium temperature in the cylinder and (b) the entropy generation during this process. What would your answer be if the piston were not free to move? Get 7.200 exercise solution
7–201 Reconsider Prob. 7–200. Using EES (or other) software, compare the results for constant specific heats to those obtained using built-in variable specific heats built into EES functions. Get 7.201 exercise solution
7–202 Repeat Prob. 7–200 by assuming the piston is made of 5 kg of copper initially at the average temperature of the two gases on both sides. Get 7.202 exercise solution
7–203 An insulated 5-m3 rigid tank contains air at 500 kPa and 57°C. A valve connected to the tank is now opened, and air is allowed to escape until the pressure inside drops to 200 kPa. The air temperature during this process is maintained constant by an electric resistance heater placed in the tank. Determine (a) the electrical energy supplied during this process and (b) the total entropy change. Get 7.203 exercise solution
7–204 In order to cool 1-ton of water at 20°C in an insulated tank, a person pours 80 kg of ice at -5°C into the water. Determine (a) the final equilibrium temperature in the tank and (b) the entropy generation during this process. The melting temperature and the heat of fusion of ice at atmospheric pressure are 0°C and 333.7 kJ/kg. Get 7.204 exercise solution
7–205 An insulated piston–cylinder device initially contains 0.02 m3 of saturated liquid–vapor mixture of water with a quality of 0.1 at 100°C. Now some ice at -18°C is dropped into the cylinder. If the cylinder contains saturated liquid at 100°C when thermal equilibrium is established, determine (a) the amount of ice added and (b) the entropy generation during this process. The melting temperature and the heat of fusion of ice at atmospheric pressure are 0°C and 333.7 kJ/kg. Get 7.205 exercise solution
7–206 Consider a 5-L evacuated rigid bottle that is surrounded by the atmosphere at 100 kPa and 17°C. A valve at the neck of the bottle is now opened and the atmospheric air is allowed to flow into the bottle. The air trapped in the bottle eventually reaches thermal equilibrium with the atmosphere as a result of heat transfer through the wall of the bottle. The valve remains open during the process so that the trapped air also reaches mechanical equilibrium with the atmosphere. Determine the net heat transfer through the wall of the bottle and the entropy generation during this filling process. Get 7.206 exercise solution
7–207 (a) Water flows through a shower head steadily at a rate of 10 L/min. An electric resistance heater placed in the water pipe heats the water from 16 to 43°C. Taking the density of water to be 1 kg/L, determine the electric power input to the heater, in kW, and the rate of entropy generation during this process, in kW/K. (b) In an effort to conserve energy, it is proposed to pass the drained warm water at a temperature of 39°C through a heat exchanger to preheat the incoming cold water. If the heat exchanger has an effectiveness of 0.50 (that is, it recovers only half of the energy that can possibly be transferred from the drained water to incoming cold water), determine the electric power input required in this case and the reduction in the rate of entropy generation in the resistance heating section. Get 7.207 exercise solution
7–208 Using EES (or other) software, determine the work input to a multistage compressor for a given set of inlet and exit pressures for any number of stages. Assume that the pressure ratio across each stage is identical and the compression process is polytropic. List and plot the compressor work against the number of stages for P1 = 100 kPa, T1 = 17°C, P2 = 800 kPa, and n = 1.35 for air. Based on this chart, can you justify using compressors with more than three stages? Get 7.208 exercise solution
7–209 A piston–cylinder device contains air that undergoes a reversible thermodynamic cycle. Initially, air is at 400 kPa and 300 K with a volume of 0.3 m3 Air is first expanded isothermally to 150 kPa, then compressed adiabatically to the initial pressure, and finally compressed at the constant pressure to the initial state. Accounting for the variation of specific heats with temperature, determine the work and heat transfer for each process. Get 7.209 exercise solution
7–210 Consider the turbocharger of an internal combustion engine. The exhaust gases enter the turbine at 450°C at a rate of 0.02 kg/s and leave at 400°C. Air enters the compressor at 70°C and 95 kPa at a rate of 0.018 kg/s and leaves at 135 kPa. The mechanical efficiency between the turbine and the compressor is 95 percent (5 percent of turbine work is lost during its transmission to the compressor). Using air properties for the exhaust gases, determine (a) the air temperature at the compressor exit and (b) the isentropic efficiency of the compressor. Get 7.210 exercise solution
7–211 Air is compressed steadily by a compressor from 100 kPa and 20°C to 1200 kPa and 300°C at a rate of 0.4 kg/s. The compressor is intentionally cooled by utilizing fins on the surface of the compressor and heat is lost from the compressor at a rate of 15 kW to the surroundings at 20°C. Using constant specific heats at room temperature, determine (a) the power input to the compressor, (b) the isothermal efficiency, and (c) the entropy generation during this process. Get 7.211 exercise solution
7–212 A 0.25-m3 insulated piston–cylinder device initially contains 0.7 kg of air at 20°C. At this state, the piston is free to move. Now air at 500 kPa and 70°C is allowed to enter the cylinder from a supply line until the volume increases by 50 percent. Using constant specific heats at room temperature, determine (a) the final temperature, (b) the amount of mass that has entered, (c) the work done, and (d) the entropy generation. Get 7.212 exercise solution
7–213 When the transportation of natural gas in a pipeline is not feasible for economic reasons, it is first liquefied using nonconventional refrigeration techniques and then transported in super-insulated tanks. In a natural gas liquefaction plant, the liquefied natural gas (LNG) enters a cryogenic turbine at 40 bar and -160°C at a rate of 55 kg/s and leaves at 3 bar. If 350 kW power is produced by the turbine, determine the efficiency of the turbine. Take the density of LNG to be 423.8 kg/m3 Get 7.213 exercise solution
7–214 Steam is condensed at a constant temperature of 30°C as it flows through the condensor of a power plant by rejecting heat at a rate of 55 MW. The rate of entropy change of steam as it flows through the condenser is (a) -1.83 MW/K (b) -0.18 MW/K (c) 0 MW/K (d) 0.56 MW/K (e) 1.22 MW/K Get 7.214 exercise solution
7–215 Steam is compressed from 6 MPa and 300°C to 10 MPa isentropically. The final temperature of the steam is (a) 290°C (b) 300°C (c) 311°C (d) 371°C (e) 422°C Get 7.215 exercise solution
7–216 An apple with an average mass of 0.15 kg and average specific heat of 3.65 kJ/kg · °C is cooled from 20°C to 5°C. The entropy change of the apple is (a) -0.0288 kJ/K (b) -0.192 kJ/K (c) -0.526 kJ/K (d) 0 kJ/K (e) 0.657 kJ/K Get 7.216 exercise solution
7–217 A piston–cylinder device contains 5 kg of saturated water vapor at 3 MPa. Now heat is rejected from the cylinder at constant pressure until the water vapor completely condenses so that the cylinder contains saturated liquid at 3 MPa at the end of the process. The entropy change of the system during this process is (a) 0 kJ/K (b) -3.5 kJ/K (c) -12.5 kJ/K (d) -17.7 kJ/K (e) -19.5 kJ/K Get 7.217 exercise solution
7–218 Helium gas is compressed from 1 atm and 25°C to a pressure of 10 atm adiabatically. The lowest temperature of helium after compression is (a) 25°C (b) 63°C (c) 250°C (d)384°C (e) 476°C Get 7.218 exercise solution
7–219 Steam expands in an adiabatic turbine from 8 MPa and 500°C to 0.1 MPa at a rate of 3 kg/s. If steam leaves the turbine as saturated vapor, the power output of the turbine is (a) 2174 kW (b) 698 kW (c) 2881 kW (d)1674 kW (e) 3240 kW Get 7.219 exercise solution
7–220 Argon gas expands in an adiabatic turbine from 3 MPa and 750°C to 0.2 MPa at a rate of 5 kg/s. The maximum power output of the turbine is (a) 1.06 MW (b) 1.29 MW (c) 1.43 MW (d)1.76 MW (e) 2.08 MW Get 7.220 exercise solution
7–221 A unit mass of a substance undergoes an irreversible process from state 1 to state 2 while gaining heat from the surroundings at temperature T in the amount of q. If the entropy of the substance is s1 at state 1, and s2 at state 2, the entropy change of the substance s during this process is

Get 7.221 exercise solution
7–222 A unit mass of an ideal gas at temperature T undergoes a reversible isothermal process from pressure P1 to pressure P2 while losing heat to the surroundings at temperature T in the amount of q. If the gas constant of the gas is R, the entropy change of the gas s during this process is

Get 7.222 exercise solution
7–223 Air is compressed from room conditions to a specified pressure in a reversible manner by two compressors: one isothermal and the other adiabatic. If the entropy change of air Dsisot during the reversible isothermal compression, and Dsadia during the reversible adiabatic compression, the correct statement regarding entropy change of air per unit mass is
Get 7.223 exercise solution
7–224 Helium gas is compressed from 15°C and 5.40 m3/kg to 0.775 m3/kg in a reversible and adiabatic manner. The temperature of helium after compression is (a) 105°C (b) 55°C (c) 1734°C (d)1051°C (e) 778°C Get 7.224 exercise solution
7–225 Heat is lost through a plane wall steadily at a rate of 600 W. If the inner and outer surface temperatures of the wall are 20°C and 5°C, respectively, the rate of entropy generation within the wall is (a) 0.11 W/K (b) 4.21 W/K (c) 2.10 W/K (d) 42.1 W/K (e) 90.0 W/K Get 7.225 exercise solution
7–226 Air is compressed steadily and adiabatically from 17°C and 90 kPa to 200°C and 400 kPa. Assuming constant specific heats for air at room temperature, the isentropic efficiency of the compressor is (a) 0.76 (b) 0.94 (c) 0.86 (d)0.84 (e) 1.00 Get 7.226 exercise solution
7–227 Argon gas expands in an adiabatic turbine steadily from 500°C and 800 kPa to 80 kPa at a rate of 2.5 kg/s. For isentropic efficiency of 80 percent, the power produced by the turbine is (a) 194 kW (b) 291 kW (c) 484 kW (d)363 kW (e) 605 kW Get 7.227 exercise solution
7–228 Water enters a pump steadily at 100 kPa at a rate of 35 L/s and leaves at 800 kPa. The flow velocities at the inlet and the exit are the same, but the pump exit where the discharge pressure is measured is 6.1 m above the inlet section. The minimum power input to the pump is (a) 34 kW (b) 22 kW (c) 27 kW (d)52 kW (e) 44 kW Get 7.228 exercise solution
7–229 Air at 15°C is compressed steadily and isothermally from 100 kPa to 700 kPa at a rate of 0.12 kg/s. The minimum power input to the compressor is (a) 1.0 kW (b) 11.2 kW (c) 25.8 kW (d)19.3 kW (e) 161 kW Get 7.229 exercise solution
7–230 Air is to be compressed steadily and isentropically from 1 atm to 25 atm by a two-stage compressor. To minimize the total compression work, the intermediate pressure between the two stages must be (a) 3 atm ( b) 5 atm ( c) 8 atm (d)10 atm (e) 13 atm Get 7.230 exercise solution
7–231 Helium gas enters an adiabatic nozzle steadily at 500°C and 600 kPa with a low velocity, and exits at a pressure of 90 kPa. The highest possible velocity of helium gas at the nozzle exit is (a) 1475 m/s (b) 1662 m/s (c) 1839 m/s (d)2066 m/s (e) 3040 m/s Get 7.231 exercise solution
7–232 Combustion gases with a specific heat ratio of 1.3 enter an adiabatic nozzle steadily at 800°C and 800 kPa with a low velocity, and exit at a pressure of 85 kPa. The lowest possible temperature of combustion gases at the nozzle exit is (a) 43°C (b) 237°C (c) 367°C (d)477°C (e) 640°C Get 7.232 exercise solution
7–233 Steam enters an adiabatic turbine steadily at 400°C and 3 MPa, and leaves at 50 kPa. The highest possible percentage of mass of steam that condenses at the turbine exit and leaves the turbine as a liquid is (a) 5% ( b) 10% (c) 15% (d)20% (e) 0% Get 7.233 exercise solution
7–234 Liquid water enters an adiabatic piping system at 15°C at a rate of 8 kg/s. If the water temperature rises by 0.2°C during flow due to friction, the rate of entropy generation in the pipe is (a) 23 W/K (b) 55 W/K (c) 68 W/K (d)220 W/K (e) 443 W/K Get 7.234 exercise solution
7–235 Liquid water is to be compressed by a pump whose isentropic efficiency is 75 percent from 0.2 MPa to 5 MPa at a rate of 0.15 m3/min. The required power input to this pump is (a) 4.8 kW (b) 6.4 kW (c) 9.0 kW (d)16.0 kW (e) 12 kW Get 7.235 exercise solution
7–236 Steam enters an adiabatic turbine at 8 MPa and 500°C at a rate of 18 kg/s, and exits at 0.2 MPa and 300°C. The rate of entropy generation in the turbine is (a) 0 kW/K (b) 7.2 kW/K (c) 21 kW/K (d)15 kW/K (e) 17 kW/K Get 7.236 exercise solution
7–237 Helium gas is compressed steadily from 90 kPa and 25°C to 600 kPa at a rate of 2 kg/min by an adiabatic compressor. If the compressor consumes 70 kW of power while operating, the isentropic efficiency of this compressor is (a) 56.7% (b) 83.7% (c) 75.4% (d)92.1% (e) 100.0% Get 7.237 exercise solution